Is Boiling A Chemical Or Physical Change
Muz Play
Mar 16, 2025 · 6 min read
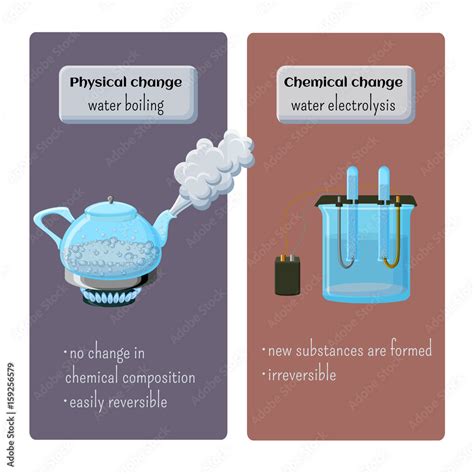
Table of Contents
Is Boiling a Chemical or Physical Change? A Deep Dive into the Science of Phase Transitions
The question of whether boiling is a chemical or physical change is a fundamental one in chemistry and physics. Understanding the difference between chemical and physical changes is crucial for grasping the nature of matter and its transformations. While seemingly simple, the process of boiling offers a rich opportunity to explore the concepts of phase transitions, intermolecular forces, and energy transfer. This article will delve deep into the science of boiling, definitively answering the question and explaining the underlying principles.
Defining Chemical and Physical Changes
Before diving into the specifics of boiling, let's establish clear definitions:
Chemical Change: A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into new substances with different chemical properties. This transformation is typically accompanied by the breaking and forming of chemical bonds, resulting in a change in the molecular structure. Key indicators of a chemical change include a change in color, odor, temperature, the formation of a precipitate, or the evolution of a gas.
Physical Change: A physical change involves a change in the physical properties of a substance without altering its chemical composition. The molecular structure remains intact. Examples include changes in state (solid, liquid, gas), shape, size, or temperature. Physical changes are generally reversible.
The Process of Boiling: A Detailed Look
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of the liquid equals the external pressure. At this point, bubbles of vapor form within the liquid and rise to the surface, escaping as gas.
Let's break down the process step-by-step:
-
Heat Absorption: When heat is applied to a liquid, the kinetic energy of its molecules increases. This means the molecules move faster and collide more frequently.
-
Overcoming Intermolecular Forces: The molecules in a liquid are held together by intermolecular forces, such as hydrogen bonds, dipole-dipole interactions, and London dispersion forces. These forces must be overcome for the liquid to transition to a gas.
-
Vaporization: As the kinetic energy of the molecules increases, some molecules gain enough energy to overcome the intermolecular forces and escape into the gaseous phase. This is called vaporization. This process can occur at any temperature, but it's significantly faster at the boiling point.
-
Bubble Formation: At the boiling point, the vapor pressure of the liquid equals the external pressure. This allows for the formation of bubbles of vapor within the liquid. These bubbles rise to the surface and burst, releasing the gaseous molecules into the atmosphere.
-
Phase Transition: Boiling represents a phase transition – a change from the liquid phase to the gaseous phase.
Why Boiling is a Physical Change
Throughout the entire boiling process, the chemical composition of the substance remains unchanged. Water, for example, remains H₂O whether it's in liquid or gaseous form. The only change is the state of matter; the molecules are simply further apart in the gaseous phase due to the increased kinetic energy. No new chemical bonds are formed, and no existing bonds are broken. The process is also reversible; the gas can be condensed back into a liquid through cooling.
Therefore, boiling is unequivocally a physical change.
Common Misconceptions
Several misconceptions often surround the nature of boiling:
-
Chemical reactions during heating: Some substances may undergo chemical decomposition at high temperatures before reaching their boiling point. For instance, certain organic compounds may decompose before boiling, resulting in the formation of new substances. This is not the boiling process itself; it's a separate chemical reaction occurring concurrently. Boiling itself, however, remains a physical change.
-
Changes in appearance: The visible changes associated with boiling, such as the formation of steam, can be misleading. The steam is still water (H₂O) in the gaseous phase; it's not a new substance.
-
Energy changes: While significant energy is required to boil a substance, the energy change is associated with a phase transition, not a chemical reaction. The energy is used to overcome intermolecular forces, not to break chemical bonds.
The Role of Pressure and Impurities
While boiling is a physical change, external factors like pressure and impurities can influence the process:
-
Pressure: The boiling point of a liquid is dependent on the external pressure. At lower pressures, the boiling point decreases, and vice versa. This is because a lower external pressure requires less vapor pressure to initiate boiling. This effect is exploited in techniques like vacuum distillation, where liquids with high boiling points are boiled at lower temperatures under reduced pressure.
-
Impurities: The presence of impurities can affect the boiling point of a liquid. Dissolved impurities usually elevate the boiling point, a phenomenon known as boiling point elevation. This is due to the interaction between the solvent and solute molecules, which requires more energy to overcome the intermolecular forces.
However, these influences don't change the fundamental nature of boiling as a physical change. The chemical composition of the substance remains unaffected; only the boiling point is altered.
Boiling vs. Other Phase Transitions
Boiling is one type of phase transition, but there are others:
-
Melting: The transition from solid to liquid. Similar to boiling, melting is also a physical change.
-
Freezing: The transition from liquid to solid. This is the reverse of melting, and also a physical change.
-
Sublimation: The transition from solid directly to gas (e.g., dry ice). This is a physical change.
-
Deposition: The transition from gas directly to solid (e.g., frost formation). This is a physical change.
All of these phase transitions involve changes in physical properties without alterations to chemical composition, confirming their status as physical changes.
Applications of Understanding Boiling as a Physical Change
Understanding that boiling is a physical change has wide-ranging applications in various fields:
-
Distillation: The process of separating liquids based on their different boiling points. This technique relies on the physical change of boiling and condensation to purify substances.
-
Cooking: Boiling is a crucial method in cooking, used to soften food, sterilize equipment, and prepare various dishes.
-
Industrial processes: Boiling is used extensively in various industrial processes, including the production of steam for power generation and the separation of components in chemical plants.
-
Scientific research: Boiling point determination is a crucial physical property used in characterizing and identifying substances in scientific research.
Conclusion: Boiling as a Physical Transformation
In conclusion, boiling is unequivocally a physical change. It involves a change in the state of matter from liquid to gas, but the chemical composition of the substance remains unchanged. While pressure and impurities can influence the boiling point, they do not alter the fundamental nature of the process as a physical transformation. Understanding this distinction is crucial for grasping the principles of chemistry, physics, and their numerous applications in various fields. The seemingly simple process of boiling provides a powerful illustration of the fundamental differences between chemical and physical changes, reinforcing our understanding of matter and its transformations. This knowledge serves as a cornerstone for further exploration of the fascinating world of phase transitions and the behavior of matter at a molecular level.
Latest Posts
Latest Posts
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
