Is Burning Gasoline A Chemical Change
Muz Play
Mar 21, 2025 · 5 min read
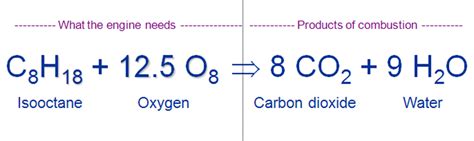
Table of Contents
Is Burning Gasoline a Chemical Change? A Deep Dive into Combustion
Burning gasoline is a quintessential example of a chemical change. While the visual spectacle of a car engine roaring to life might seem straightforward, the underlying chemical reactions are surprisingly complex and fascinating. This article will delve deep into the science behind burning gasoline, explaining why it's undeniably a chemical change and exploring the implications of this process.
Understanding Chemical Changes
Before examining gasoline combustion, let's establish a clear understanding of what constitutes a chemical change. A chemical change, also known as a chemical reaction, involves the rearrangement of atoms to form new substances with different properties. Crucially, this rearrangement is irreversible—you can't simply undo the process to recover the original materials. In contrast, a physical change alters the form or appearance of a substance without changing its chemical composition. Think of melting ice—it changes from solid to liquid, but it remains water (H₂O).
Key characteristics of chemical changes include:
- Formation of new substances: The products have different chemical properties and compositions than the reactants.
- Irreversibility: The original substances cannot be easily recovered.
- Energy changes: Chemical reactions usually involve either the release (exothermic) or absorption (endothermic) of energy.
- Observable changes: These changes might include color change, gas production, precipitate formation, or temperature change.
The Chemistry of Gasoline
Gasoline isn't a single compound but a complex mixture of hydrocarbons, primarily alkanes, with varying chain lengths. These hydrocarbons are organic molecules consisting solely of carbon and hydrogen atoms. The most common components range from 4 to 12 carbon atoms per molecule (e.g., butane, octane, decane). These molecules are relatively stable under normal conditions, but their reactivity drastically increases when exposed to the right conditions – ignition.
Octane Rating and Engine Performance
The octane rating of gasoline reflects its resistance to knocking (pre-ignition) during combustion in an internal combustion engine. A higher octane rating means the fuel is less prone to knocking, allowing for higher compression ratios and improved engine performance. This is because different hydrocarbon molecules ignite at slightly different temperatures and pressures.
The Combustion Process: A Chemical Reaction in Action
The burning of gasoline is a rapid oxidation reaction, a type of chemical reaction where a substance reacts with oxygen. In the context of an internal combustion engine, this reaction is carefully controlled and ignited by a spark plug. The overall chemical equation for the complete combustion of octane (a major component of gasoline), is:
2C₈H₁₈ + 25O₂ → 16CO₂ + 18H₂O + Energy
This equation shows that octane (C₈H₁₈) reacts with oxygen (O₂) to produce carbon dioxide (CO₂), water (H₂O), and a significant amount of energy. This energy is what powers the engine, driving the pistons and ultimately propelling the vehicle.
Incomplete Combustion: A Less Desirable Outcome
The complete combustion equation represents an ideal scenario. In reality, incomplete combustion often occurs, especially under conditions of insufficient oxygen or low temperatures. Incomplete combustion produces harmful byproducts such as carbon monoxide (CO) and soot (unburnt carbon particles).
Incomplete combustion reactions can be represented by equations such as:
- 2C₈H₁₈ + 17O₂ → 16CO + 18H₂O (Producing carbon monoxide)
- C₈H₁₈ + 9O₂ → 8C + 9H₂O (Producing soot)
These incomplete combustion products are toxic and contribute to air pollution. Catalytic converters in modern vehicles are designed to mitigate these harmful emissions by converting CO and unburnt hydrocarbons into less harmful substances like CO₂ and H₂O.
Evidence Supporting Chemical Change
Several key observations unequivocally demonstrate that burning gasoline is a chemical change:
- Formation of new substances: The products, CO₂, H₂O, and energy, are distinctly different from the reactants, gasoline and oxygen. The original gasoline is irreversibly transformed.
- Irreversibility: You cannot easily recover the original gasoline from the combustion products. The process is chemically irreversible.
- Energy release: The combustion of gasoline is highly exothermic, releasing a large amount of energy in the form of heat and light. This energy is harnessed to do work in an internal combustion engine.
- Observable changes: The burning of gasoline involves a visible flame, the production of heat, and the release of gases. These are all indicators of a chemical reaction.
- Change in chemical properties: The original gasoline is a flammable liquid with distinct chemical properties. The products of combustion, CO2 and H2O, are non-flammable gases with completely different properties.
The Role of Activation Energy
For the combustion reaction to occur, a certain amount of energy, called activation energy, is needed to initiate the process. This energy overcomes the initial energy barrier preventing the reaction from starting spontaneously. In a car engine, the spark plug provides the necessary activation energy to initiate the rapid oxidation of gasoline. Once the reaction starts, the energy released sustains the process in a chain reaction.
Environmental Implications of Gasoline Combustion
The widespread use of gasoline for transportation has significant environmental consequences. While the combustion of gasoline produces CO₂ and H₂O, the production of CO₂ contributes significantly to the greenhouse effect and global warming. Incomplete combustion produces harmful pollutants that contribute to air and water pollution, negatively affecting human health and the environment. Therefore, ongoing research focuses on developing alternative fuels and more efficient combustion technologies to reduce the environmental impact of gasoline combustion.
Conclusion: Burning Gasoline - Irrefutably a Chemical Change
The burning of gasoline is undeniably a chemical change. The formation of new substances, the irreversibility of the process, the release of energy, and the observable changes all firmly support this conclusion. Understanding the chemistry behind this process is crucial not only for appreciating the workings of internal combustion engines but also for addressing the environmental challenges associated with gasoline consumption and developing sustainable alternatives. By continuing to study and improve combustion processes, we can aim for a future with cleaner and more efficient energy technologies. The study of gasoline combustion serves as an excellent case study for demonstrating the fundamental principles of chemical reactions and their practical applications and consequences. Further research into cleaner burning fuels and more efficient combustion techniques is imperative for mitigating the negative environmental impact of gasoline-powered vehicles. The pursuit of sustainable alternatives remains a significant challenge and opportunity for the future of transportation and energy production.
Latest Posts
Latest Posts
-
Find An Equation Of The Tangent Plane To The Surface
Mar 22, 2025
-
How To Find The Coordination Number
Mar 22, 2025
-
Functional Unit Of The Kidney Is
Mar 22, 2025
-
Do Fungi Reproduce Asexually Or Sexually
Mar 22, 2025
-
Who Identified Psychological Disorders As A Harmful Dysfunction
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Burning Gasoline A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
