Is Cn A Good Leaving Group
Muz Play
Mar 31, 2025 · 5 min read
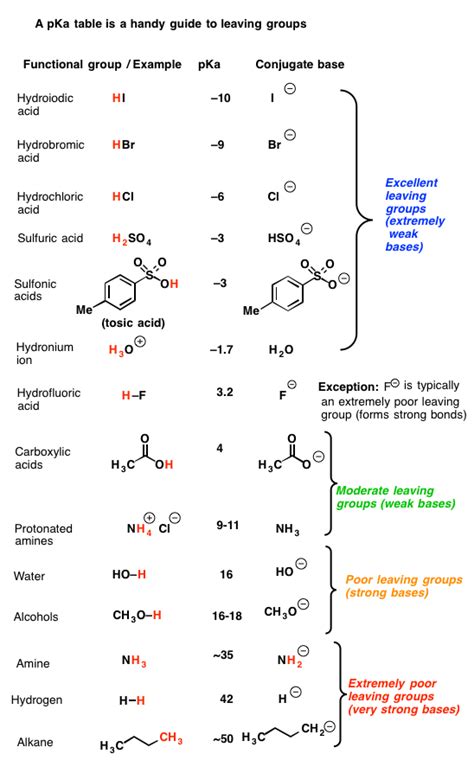
Table of Contents
Is CN⁻ a Good Leaving Group? A Deep Dive into Nucleophilic and Leaving Group Behavior
The question of whether the cyanide ion (CN⁻) is a good leaving group is a complex one, not easily answered with a simple "yes" or "no." Its behavior is highly dependent on the specific reaction conditions and the nature of the substrate. This article will explore the factors influencing CN⁻'s leaving group ability, comparing it to other common leaving groups, and analyzing its role in various reaction mechanisms. We'll delve into the nuances of its nucleophilicity and its relative leaving group aptitude, providing a comprehensive understanding of its behavior in organic chemistry.
Understanding Leaving Group Ability
Before examining CN⁻ specifically, let's establish the criteria for a good leaving group. A good leaving group is a species that can stabilize the negative charge it acquires after leaving the molecule. This stability is crucial because the departure of the leaving group generates a transition state with a partial negative charge on the leaving group. The better it can accommodate this charge, the lower the activation energy of the reaction, leading to a faster reaction rate. Key factors determining leaving group ability include:
- Stability of the conjugate base: The more stable the conjugate base (the leaving group after it departs), the better the leaving group. Factors contributing to stability include resonance, electronegativity, and size.
- Weak basicity: Good leaving groups are generally weak bases. Strong bases are reluctant to leave because they strongly prefer to hold onto their electrons. A weak base readily accepts the positive charge left behind when the bond breaks.
- Polarizability: Larger, more polarizable leaving groups are generally better because they can better distribute the negative charge.
CN⁻: A Nucleophile First and Foremost
CN⁻ is famously known for its strong nucleophilicity. This is due to the presence of the highly electronegative nitrogen atom and the lone pair of electrons on the carbon atom. This makes it a potent attacker in nucleophilic substitution (SN1 and SN2) reactions and addition reactions. Its strong nucleophilicity often overshadows its potential as a leaving group.
Nucleophilic Attacks and Competition:
In many reactions where CN⁻ could potentially act as a leaving group, it's more likely to act as a nucleophile. For instance, in an SN2 reaction with an alkyl halide, CN⁻ would readily attack the carbon atom, displacing the halide ion. The halide, in this case, acts as a better leaving group than CN⁻.
Examples of CN⁻ as a Nucleophile:
- Cyanohydrin formation: CN⁻ readily adds to carbonyl compounds (aldehydes and ketones) to form cyanohydrins. Here, CN⁻ acts exclusively as a nucleophile.
- Nitrile synthesis: Alkyl halides react with CN⁻ to form nitriles. Again, CN⁻ is the nucleophile, and the halide is the leaving group.
CN⁻ as a Leaving Group: Exceptional Circumstances
While not typically considered a good leaving group, CN⁻ can act as one under specific, often harsh, conditions. These conditions generally involve making the leaving group a weaker base or otherwise stabilizing its negative charge after it departs.
Conditions Favoring CN⁻ as a Leaving Group:
- Acidic conditions: Protonation of the nitrogen atom in CN⁻ weakens its basicity, making it a better leaving group. This protonation reduces the electron density on the nitrogen and therefore improves its leaving ability.
- Formation of a stable carbocation: If the reaction involves the formation of a particularly stable carbocation (e.g., tertiary carbocation, benzylic carbocation, allylic carbocation), the leaving of CN⁻ becomes more favorable. The stability of the carbocation helps to offset the less favorable nature of CN⁻ as a leaving group.
- Specific reaction mechanisms: Certain reaction mechanisms might favor CN⁻ as a leaving group due to specific steric or electronic factors that may not be relevant in other scenarios.
Examples of CN⁻ as a Leaving Group (rare):
The instances where CN⁻ acts as a leaving group are usually limited to specialized reactions or circumstances and are less frequently encountered in standard organic chemistry. It's crucial to remember that the reaction conditions are highly specialized and often involve harsh conditions not typically favorable for general organic reactions. A detailed mechanistic understanding is necessary to predict its behavior.
Comparison with Other Leaving Groups
Let's compare CN⁻ with some common leaving groups:
| Leaving Group | Basicity | Stability of Conjugate Acid | Leaving Group Ability |
|---|---|---|---|
| I⁻ (iodide) | Very Weak | Very Stable | Excellent |
| Br⁻ (bromide) | Weak | Stable | Good |
| Cl⁻ (chloride) | Weak | Stable | Moderate |
| H₂O (water) | Weak | Stable | Moderate |
| Tosylate (OTs) | Very Weak | Very Stable | Excellent |
| Mesylate (OMs) | Very Weak | Very Stable | Excellent |
| CN⁻ (cyanide) | Moderate | Moderately Stable (depending on conditions) | Poor (except under specific conditions) |
As the table shows, CN⁻ is significantly weaker as a leaving group compared to halides or sulfonate esters. Its moderate basicity and relatively unstable conjugate acid hinder its ability to leave readily.
Conclusion: Context is Key
The leaving group ability of CN⁻ is highly context-dependent. While its strong nucleophilicity often dominates its behavior in reactions, it can act as a leaving group under specific and often harsh conditions, such as highly acidic environments or reactions that generate highly stable carbocations. Its performance as a leaving group is significantly inferior to common leaving groups like halides and sulfonate esters. Therefore, while theoretically possible, expecting CN⁻ to function as a good leaving group is unlikely in most typical organic chemistry scenarios. Understanding the specific reaction conditions, mechanism, and the relative stability of potential intermediates is crucial for predicting whether CN⁻ will act as a nucleophile or a leaving group. Its behaviour is best interpreted through a careful analysis of the particular reaction at hand. Its role as a nucleophile remains far more common and readily apparent than its rare occurrences as a leaving group.
Latest Posts
Latest Posts
-
External Parts Of A Computer System
Apr 01, 2025
-
Is Covalent Bond Between Two Nonmetals
Apr 01, 2025
-
Do Porifera Have A Digestive System
Apr 01, 2025
-
Competing Visions A History Of California
Apr 01, 2025
-
What Are Three Main Ideas Of The Cell Theory
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Cn A Good Leaving Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
