Is Coffee An Element Compound Or Mixture
Muz Play
Mar 20, 2025 · 6 min read
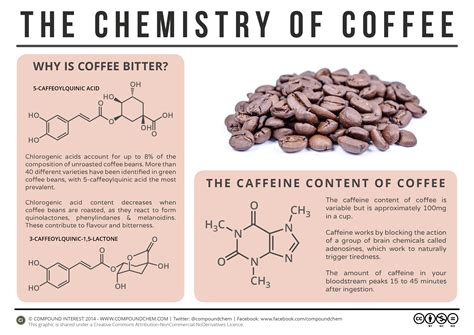
Table of Contents
Is Coffee an Element, Compound, or Mixture? A Deep Dive into the Chemistry of Your Morning Brew
Coffee, that aromatic elixir that jumpstarts millions of mornings worldwide, is more than just a delicious beverage. It's a complex concoction of various chemical substances, presenting a fascinating case study in chemistry. The question, "Is coffee an element, compound, or mixture?" has a straightforward answer, but unpacking the "why" behind that answer reveals a rich tapestry of chemical components and processes.
Understanding the Basics: Elements, Compounds, and Mixtures
Before we delve into the specifics of coffee, let's establish a clear understanding of the three fundamental classifications of matter:
Elements:
Elements are the simplest form of matter. They are pure substances made up of only one type of atom. Think of hydrogen (H), oxygen (O), carbon (C), and gold (Au) – these are all elements, represented on the periodic table. They cannot be broken down into simpler substances by chemical means.
Compounds:
Compounds are substances formed when two or more elements chemically combine in a fixed ratio. This combination results in a new substance with properties different from its constituent elements. Water (H₂O), for example, is a compound formed from two hydrogen atoms and one oxygen atom. Compounds can be broken down into their constituent elements through chemical reactions.
Mixtures:
Mixtures are combinations of two or more substances that are not chemically bonded. The substances retain their individual properties and can be separated by physical methods like filtration, distillation, or evaporation. Examples include saltwater (salt and water), air (various gases), and, you guessed it, coffee.
The Verdict: Coffee is a Mixture
The answer is clear: coffee is a mixture. It's a complex blend of numerous chemical compounds, each retaining its own properties and not chemically bonded to each other. These compounds are derived from the coffee bean itself and the various processes involved in making a cup of coffee.
The Chemical Components of Coffee: A Closer Look
Let's explore the diverse array of chemical compounds that contribute to coffee's rich and complex profile:
1. Water:
The most abundant component in brewed coffee is water, acting as the solvent that extracts the flavorful and aromatic compounds from the coffee grounds. Water itself is a compound (H₂O).
2. Caffeine:
Caffeine (C₈H₁₀N₄O₂) is arguably the most well-known component of coffee, acting as a central nervous system stimulant. It's an alkaloid, a naturally occurring organic compound containing nitrogen. Caffeine is a compound, and a significant contributor to coffee's effects on the body.
3. Acids:
Coffee contains several organic acids, contributing significantly to its characteristic taste and aroma. These include:
- Chlorogenic acids: These acids are responsible for much of coffee's antioxidant properties and bitterness. They are polyphenols, a type of compound known for their potential health benefits.
- Acetic acid: This contributes to the sourness in coffee.
- Citric acid: Another acid that adds to the overall acidity profile of coffee.
- Quinic acid: A component of chlorogenic acids.
Each of these acids is a distinct compound.
4. Sugars:
Coffee beans contain various sugars, including sucrose, glucose, and fructose. These sugars contribute to the sweetness and body of the coffee. Sugars are compounds.
5. Aromatic Compounds:
Hundreds of volatile aromatic compounds contribute to the diverse and nuanced aroma of coffee. These compounds are responsible for the rich olfactory experience associated with a freshly brewed cup. They include:
- Esters: These contribute fruity and floral notes.
- Aldehydes: These can contribute grassy or citrusy notes.
- Ketones: These offer sweet and fruity aromas.
- Alcohols: These can add to the overall complexity of the aroma.
Each of these aromatic compounds is an individual chemical compound.
6. Lipids and Oils:
Coffee beans also contain various lipids and oils, contributing to the mouthfeel and aroma. These are complex mixtures of different molecules, not single compounds.
7. Minerals:
Coffee beans contain small amounts of minerals like potassium, magnesium, and phosphorus. These are elements, but in the context of the brewed coffee, they're part of the overall mixture.
The Brewing Process: A Mixture Transformation
The brewing process itself is a crucial step in determining the final composition of coffee. It's a process of extraction, where water dissolves many of the compounds present in the coffee grounds, resulting in a more concentrated mixture of these compounds. The precise composition of the brewed coffee will depend on factors like:
- Grind size: A finer grind will increase the surface area of the coffee grounds and lead to a more concentrated brew.
- Water temperature: Different temperatures will extract different compounds.
- Brewing time: Longer brewing times can lead to over-extraction and bitterness.
- Coffee-to-water ratio: This affects the concentration of the final brew.
These variables demonstrate that even though coffee starts as a mixture of compounds, the brewing process transforms the balance and concentration of these compounds in the final cup.
Beyond the Basics: Exploring the Complexity
The complexity of coffee extends beyond the chemical compounds mentioned above. Factors like the type of bean (Arabica, Robusta, etc.), roasting level, and geographical origin significantly affect the final chemical composition and sensory properties of the brew. Each factor influences the levels of various compounds, creating an almost limitless array of flavor profiles and aroma characteristics.
For example, a dark roast coffee will have different concentrations of acids and aromatic compounds compared to a light roast due to the chemical changes that occur during the roasting process. This is a purely physical change in the compounds, not a transformation from one type of matter to another.
Coffee as a System: Understanding the Interactions
It's crucial to understand that while coffee is a mixture, the components within it do interact with each other. These interactions affect the overall taste, aroma, and mouthfeel. For example, the interaction between acids and other compounds can influence the perceived bitterness and sourness. Similarly, the interaction between aromatic compounds and lipids can affect the perception of aroma and body.
Conclusion: A Complex and Delightful Mixture
In conclusion, coffee is unequivocally a mixture. It's a complex and dynamic blend of various chemical compounds, each contributing to its unique properties. Understanding the chemical composition of coffee allows us to appreciate the intricacies of this beloved beverage and appreciate the science behind the perfect cup. The brewing process is a transformation within the existing mixture, altering the relative abundance of its components. The ongoing research into coffee chemistry continues to unveil new discoveries, further demonstrating the fascinating and complex nature of this everyday drink. The next time you savor a cup, take a moment to appreciate the intricate dance of molecules that makes your morning brew so uniquely enjoyable.
Latest Posts
Latest Posts
-
Limiting Reactant And Percent Yield Practice
Mar 21, 2025
-
The Long Love That In My Thought Doth Harbor
Mar 21, 2025
-
Function Of The Stage Of A Microscope
Mar 21, 2025
-
Periodic Table Solids Liquids And Gases
Mar 21, 2025
-
Coefficient Of Performance For Refrigeration Cycle
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is Coffee An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
