Periodic Table Solids Liquids And Gases
Muz Play
Mar 21, 2025 · 6 min read
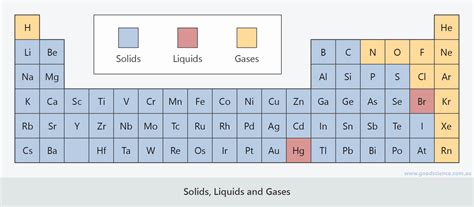
Table of Contents
The Periodic Table: A Deep Dive into Solids, Liquids, and Gases
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While it primarily focuses on atomic structure and reactivity, it also offers invaluable insight into the physical states of matter: solid, liquid, and gas. Understanding the relationship between an element's position on the table and its state at a given temperature and pressure is crucial to comprehending its behavior and applications. This comprehensive exploration delves into the fascinating interplay between the periodic table and the three fundamental states of matter.
The Influence of Atomic Structure on Physical State
The state of matter an element exists in is primarily determined by the strength of the intermolecular forces between its atoms or molecules. These forces, in turn, are heavily influenced by the element's atomic structure, which is reflected in its position on the periodic table.
Metals: A Kingdom of Solids
The majority of elements on the periodic table are metals, and overwhelmingly, they exist as solids at room temperature. This is due to the strong metallic bonding between their atoms. Metallic bonding arises from the delocalization of valence electrons, creating a "sea" of electrons that surrounds positively charged metal ions. This strong electrostatic attraction holds the metal atoms tightly together, resulting in a rigid, crystalline structure characteristic of solids. The strength of this metallic bond varies, accounting for differences in melting points across metals. For instance, tungsten (W), with its exceptionally high melting point, exhibits exceptionally strong metallic bonding, while mercury (Hg), a liquid at room temperature, has relatively weaker metallic bonds.
Non-metals: A Diverse Trio
Non-metals occupy the upper right corner of the periodic table and exhibit a far greater diversity in their physical states at room temperature.
Gaseous Non-Metals:
Many non-metals exist as gases at room temperature. This is because their atoms form weak intermolecular forces, primarily van der Waals forces. These weak forces are insufficient to hold the atoms together in a solid or liquid state at normal temperatures. Examples include:
- Hydrogen (H): The smallest and lightest element, forming diatomic molecules (H₂) with weak London dispersion forces.
- Oxygen (O): Crucial for respiration, oxygen also forms diatomic molecules (O₂) with relatively weak van der Waals forces.
- Nitrogen (N): The most abundant element in the atmosphere, existing as diatomic molecules (N₂) with similarly weak van der Waals forces.
- Fluorine (F) and Chlorine (Cl): These halogens also exist as diatomic gases due to weak intermolecular forces.
Solid Non-Metals:
Some non-metals, however, exist as solids at room temperature. This is due to stronger intermolecular forces, such as covalent bonding within the molecules themselves and weaker intermolecular forces between these molecules. Examples include:
- Carbon (C): Exists in various allotropes, including diamond and graphite, where strong covalent bonds within the structures lead to solid states. Diamond boasts a three-dimensional network of strong covalent bonds, resulting in its extreme hardness. Graphite, on the other hand, features layered structures with weaker intermolecular forces between layers, accounting for its softness and lubricating properties.
- Sulfur (S): Forms various allotropes, most commonly a yellow, crystalline solid with relatively strong covalent bonds within the S8 molecules and weaker van der Waals forces between them.
- Phosphorus (P): Exists in several allotropes, with white phosphorus being a highly reactive solid composed of P₄ tetrahedra held together by weak van der Waals forces. Red phosphorus, another allotrope, is a polymeric form with stronger covalent bonds.
- Iodine (I): Exists as a dark purple crystalline solid with strong covalent bonds within I₂ molecules, but weaker intermolecular forces between these molecules.
Liquid Non-Metals:
Bromine (Br) is the only non-metal that exists as a liquid at room temperature. While it forms diatomic molecules (Br₂), the relatively strong van der Waals forces between these molecules are sufficient to keep it in a liquid state under normal conditions.
Metalloids: A Bridge Between States
Metalloids, situated between metals and non-metals on the periodic table, exhibit properties intermediate between the two. Their physical states at room temperature are varied. Silicon (Si) and Germanium (Ge) are solids, while Boron (B) is also a solid, highlighting the diverse behavior of this group.
Temperature and Pressure: Modifying the State
While the inherent properties of an element strongly influence its state, temperature and pressure play a crucial role in determining whether an element exists as a solid, liquid, or gas.
Temperature's Influence:
Increasing temperature provides atoms or molecules with more kinetic energy, overcoming the intermolecular forces holding them together. This leads to a phase transition:
- Solid to Liquid (Melting): Sufficient energy overcomes the strong forces in the solid state, allowing atoms or molecules to move more freely.
- Liquid to Gas (Boiling/Vaporization): Even more energy is required to completely overcome the intermolecular forces, leading to a transition to the gaseous state where atoms or molecules are largely independent.
Pressure's Influence:
Pressure affects the proximity of atoms or molecules. Increasing pressure forces them closer together, strengthening intermolecular forces and favouring the condensed phases (solid and liquid). Conversely, decreasing pressure allows atoms or molecules to spread out, weakening intermolecular forces and favoring the gaseous phase.
Phase Diagrams: Visualizing State Changes
Phase diagrams provide a visual representation of the state of an element or compound as a function of temperature and pressure. These diagrams show the boundaries between solid, liquid, and gaseous phases, as well as points where multiple phases coexist (e.g., the triple point where all three phases coexist in equilibrium). Analyzing a phase diagram for a specific element allows prediction of its state under various temperature and pressure conditions.
Applications Based on Physical State
The physical state of an element significantly impacts its applications. The solid state is often utilized for structural applications (e.g., iron in construction), while gases are utilized in various industrial processes (e.g., oxygen in welding). Liquids are used in a wide array of applications, from solvents to coolants. The versatility of elements across different states highlights the richness and significance of understanding the periodic table's relationship to physical states of matter.
Conclusion: A Unified Perspective
The periodic table, while primarily organized by atomic structure and chemical properties, offers a powerful framework for understanding the physical states of matter. The strength of intermolecular forces, largely determined by an element's position and atomic structure, plays a dominant role in determining whether an element is a solid, liquid, or gas at room temperature. However, temperature and pressure significantly influence phase transitions, leading to the diverse array of applications for elements in their various states. Mastering this connection between the periodic table and states of matter is fundamental for appreciating the multifaceted nature of chemistry and its vast array of applications in the world around us. Further research into the complex interplay between these factors opens doors to technological advancements and a deeper understanding of our physical world. The periodic table, a seemingly simple chart, thus unlocks a universe of knowledge and possibilities.
Latest Posts
Latest Posts
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
-
Song Lyrics Ode To Billy Joe
Mar 27, 2025
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Solids Liquids And Gases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
