Is Color Change A Physical Or Chemical Change
Muz Play
Mar 28, 2025 · 7 min read
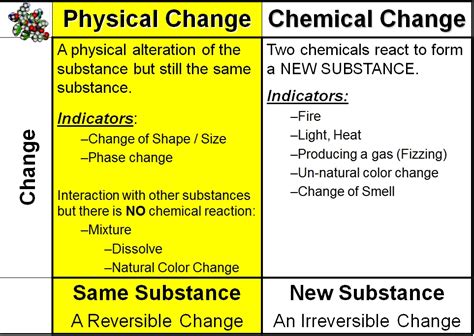
Table of Contents
Is a Color Change a Physical or Chemical Change? A Comprehensive Guide
The question of whether a color change signifies a physical or chemical change is a common one, especially in science education. The simple answer is: it depends. A color change alone isn't sufficient to definitively classify a change as physical or chemical. While many chemical changes do involve color changes, many physical changes also exhibit shifts in hue, tint, or shade. Understanding the nuances requires a deeper dive into the underlying processes.
Understanding Physical and Chemical Changes
Before tackling the complexities of color change, let's establish a clear understanding of the core differences between physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance without changing its chemical composition. The molecules themselves remain unchanged; they simply rearrange or are separated. Examples include:
- Changes in state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid). These changes involve alterations in the arrangement of water molecules but don't change the water molecules themselves.
- Dissolving: Dissolving sugar in water changes the appearance of the mixture, but the sugar molecules remain intact. You can retrieve the sugar through evaporation.
- Crushing: Crushing a rock changes its size and shape, but it's still the same rock chemically.
- Cutting: Cutting paper alters its size but doesn't change its chemical composition.
Key characteristics of physical changes:
- No new substances are formed.
- Changes are often reversible.
- Changes involve relatively small energy changes.
Chemical Changes (Chemical Reactions)
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different chemical properties. These changes often involve the breaking and forming of chemical bonds. Examples include:
- Burning: Burning wood involves a reaction with oxygen, creating ash, smoke, and gases (carbon dioxide, water vapor). The original wood is transformed into entirely different substances.
- Rusting: Rusting iron involves a reaction with oxygen and water, forming iron oxide (rust), a new substance with different properties.
- Cooking: Cooking an egg alters its chemical composition, making it irreversibly different from a raw egg.
- Digestion: The digestive process breaks down food molecules into simpler substances, a series of chemical reactions.
Key characteristics of chemical changes:
- New substances are formed with different properties.
- Changes are often irreversible.
- Changes often involve significant energy changes (heat, light, etc.).
Color Change as an Indicator
While a color change isn't a foolproof indicator of a chemical reaction, it's often a strong clue. Let's examine examples where color change accompanies both physical and chemical changes:
Color Changes in Physical Changes
Several physical changes involve color alterations without altering the chemical makeup:
- Dissolving colored substances: Dissolving a colored substance like food coloring in water changes the appearance of the water, but the food coloring molecules remain intact. The color dilution is a physical change.
- Mixing substances: Mixing different colored substances can result in a new color through simple blending, not a chemical reaction. Think of mixing blue and yellow paint to make green. This is a physical, not a chemical, change.
- Changes in state (some instances): While typically not involving major color shifts, the formation of certain crystals from a solution can sometimes present a different color than the solution itself due to how light interacts with the crystal structure. This is largely a physical change, although subtle shifts in electronic configurations might be responsible.
- Light scattering: The color you observe is often due to light interacting with a material. Changes in the size and arrangement of particles can affect light scattering, leading to perceived color changes. Think of the way light scatters differently through different sized colloidal suspensions. This is a physical phenomenon.
Color Changes in Chemical Changes
Many chemical reactions are accompanied by dramatic color changes:
- Oxidation: The rusting of iron (oxidation) is a chemical change resulting in a brown color. The iron reacts with oxygen to form iron oxide (rust), a completely new substance.
- Reactions involving transition metals: Many transition metal compounds exhibit vibrant colors due to the electronic configurations of their ions. Reactions involving these metals often produce striking color changes. For example, the reaction of copper(II) sulfate with iron produces a color change indicative of a chemical transformation.
- Acid-base reactions: Certain acid-base reactions, particularly those involving indicators like phenolphthalein, cause distinct color changes depending on pH. The change in color reflects a chemical transformation, not just a simple mixing.
- Combustion: Many combustion reactions produce a color change. For instance, the burning of wood causes a change from brown to ash grey, a clear indication of a chemical reaction.
How to Determine if a Color Change is Physical or Chemical
Distinguishing between physical and chemical color changes often requires considering additional factors beyond just the visual observation. Look for these indicators:
- Reversibility: Can the original color be restored easily? If yes, it's more likely to be a physical change (like diluting a colored solution). If not, it may indicate a chemical change.
- Temperature changes: Significant temperature changes, including the production or absorption of heat (exothermic or endothermic reactions), often accompany chemical changes.
- Gas evolution: The production of bubbles or gases is a strong indicator of a chemical reaction.
- Formation of a precipitate: The formation of a solid from a solution (precipitate) indicates a chemical reaction.
- New properties: Does the substance exhibit new properties (e.g., flammability, reactivity, solubility) after the color change? This suggests a chemical change.
Careful Observation and Experimental Design: The key to determining whether a color change is physical or chemical is through meticulous observation and, ideally, controlled experimentation. You might need to perform additional tests to analyze the composition of the substance before and after the color change.
Examples of Color Change Scenarios
Let's analyze a few specific examples to clarify the distinctions:
Scenario 1: Dissolving Potassium Permanganate
Dissolving potassium permanganate (KMnO₄) in water results in a deep purple solution. This is a physical change. The KMnO₄ molecules remain intact; they are simply dispersed in the water. The purple color is due to the absorption of specific wavelengths of light by the permanganate ions. Evaporating the water will recover the original purple solid.
Scenario 2: Burning Magnesium
Burning magnesium ribbon produces a bright white light and a white powder (magnesium oxide). This is a chemical change. Magnesium reacts with oxygen in the air, forming magnesium oxide, a completely new substance with different properties. The bright light and formation of a white powder are clear indications of a chemical reaction.
Scenario 3: Mixing Blue and Yellow Paint
Mixing blue and yellow paint produces green paint. This is a physical change. No new chemical substances are formed; the colors simply mix to create a different visual effect. The original colors can be separated through careful techniques, demonstrating the reversible nature of this change.
Scenario 4: Adding Iodine to Starch
Adding iodine solution to starch results in a dark blue-black color. This is a chemical change. While not a dramatic reaction, the iodine forms a complex with the amylose component of the starch, altering its chemical properties and resulting in a new color.
Conclusion
Determining whether a color change signals a physical or chemical transformation necessitates careful consideration of the accompanying phenomena. While color alteration often accompanies chemical reactions, it's not a definitive criterion. The key lies in examining the reversibility of the change, presence of heat or gas, formation of precipitates, and emergence of new chemical properties. Through thorough observation and, when necessary, complementary experiments, one can accurately classify whether a color change represents a physical or chemical alteration. Understanding these distinctions is essential for grasping the fundamental principles of chemistry and the nature of matter itself.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Uniform Rod
Mar 31, 2025
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
-
What Is The Chemical Equation For Aerobic Respiration
Mar 31, 2025
-
Equation Of The Tangent Line Implicit Differentiation
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Color Change A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
