Is Formal Charge The Same As Oxidation Number
Muz Play
Mar 19, 2025 · 5 min read
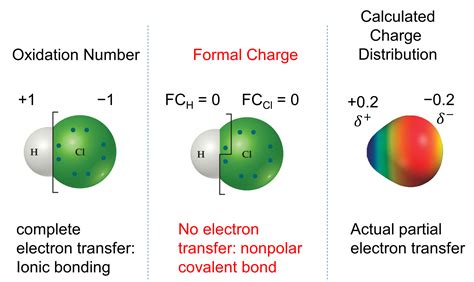
Table of Contents
Is Formal Charge the Same as Oxidation Number? A Deep Dive into Chemical Concepts
Understanding chemical bonding and electron distribution is crucial in chemistry. Two key concepts often used in this context are formal charge and oxidation number. While both relate to electron assignment in a molecule or ion, they represent different aspects and are calculated differently. This comprehensive article will explore the nuances of formal charge and oxidation number, highlighting their similarities and crucial differences, and providing clear examples to solidify your understanding.
What is Formal Charge?
Formal charge is a bookkeeping tool used to determine the distribution of electrons within a molecule or polyatomic ion. It helps predict the most plausible Lewis structure when multiple possibilities exist. The formal charge is not a real charge; it's a hypothetical charge assigned to an atom assuming equal sharing of electrons in a covalent bond.
Calculating Formal Charge:
The formula for calculating formal charge is straightforward:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)
Where:
- Valence Electrons: The number of electrons an atom typically has in its outermost shell.
- Non-bonding Electrons: The number of electrons that are not involved in any covalent bonds (lone pairs).
- Bonding Electrons: The number of electrons involved in covalent bonds.
Example of Formal Charge Calculation:
Let's consider the carbonate ion (CO₃²⁻). One possible Lewis structure shows one double bond and two single bonds from the carbon atom to the oxygen atoms.
-
Carbon:
- Valence electrons: 4
- Non-bonding electrons: 0
- Bonding electrons: 8 (4 bonds x 2 electrons/bond)
- Formal charge = 4 - 0 - (1/2 * 8) = 0
-
Oxygen (double bonded):
- Valence electrons: 6
- Non-bonding electrons: 4
- Bonding electrons: 4
- Formal charge = 6 - 4 - (1/2 * 4) = 0
-
Oxygen (single bonded):
- Valence electrons: 6
- Non-bonding electrons: 6
- Bonding electrons: 2
- Formal charge = 6 - 6 - (1/2 * 2) = -1
In this structure, two oxygen atoms have a formal charge of -1, and the carbon and the other oxygen have a formal charge of 0. The sum of formal charges equals the overall charge of the ion (-2).
What is Oxidation Number?
Oxidation number (also called oxidation state) is a different concept that reflects the relative charge on an atom in a molecule or ion. It represents the number of electrons an atom has gained or lost compared to its neutral state. Unlike formal charge, oxidation number accounts for electronegativity differences between atoms and assumes that electrons are completely transferred to the more electronegative atom in a bond.
Assigning Oxidation Numbers:
Several rules guide the assignment of oxidation numbers:
-
Free elements: The oxidation number of an atom in its elemental form is always 0. (e.g., O₂ , Na)
-
Monatomic ions: The oxidation number of a monatomic ion is equal to its charge. (e.g., Na⁺ = +1, Cl⁻ = -1)
-
Fluorine: Fluorine always has an oxidation number of -1 in its compounds.
-
Oxygen: Oxygen usually has an oxidation number of -2, except in peroxides (e.g., H₂O₂) where it's -1 and in compounds with fluorine where it can be positive.
-
Hydrogen: Hydrogen usually has an oxidation number of +1, except in metal hydrides (e.g., NaH) where it's -1.
-
Group 1 elements: Always have an oxidation number of +1.
-
Group 2 elements: Always have an oxidation number of +2.
-
The sum of oxidation numbers: In a neutral molecule, the sum of all oxidation numbers must be zero. In a polyatomic ion, the sum of oxidation numbers equals the charge of the ion.
Example of Oxidation Number Calculation:
Let's use the same carbonate ion (CO₃²⁻) example:
-
Oxygen: Each oxygen atom has an oxidation number of -2 (following rule 4).
-
Carbon: Let x be the oxidation number of carbon. The sum of oxidation numbers must equal the charge of the ion: x + 3(-2) = -2 x = +4
Therefore, the oxidation number of carbon in CO₃²⁻ is +4.
Key Differences between Formal Charge and Oxidation Number:
The fundamental distinction lies in their underlying assumptions and applications:
| Feature | Formal Charge | Oxidation Number |
|---|---|---|
| Assumption | Equal sharing of electrons in a covalent bond | Complete transfer of electrons |
| Electronegativity | Ignores electronegativity differences | Considers electronegativity differences |
| Calculation | Based on valence electrons and bond distribution | Based on assigned rules and electronegativity |
| Purpose | Predicting the most plausible Lewis structure | Determining the relative charge and electron distribution, tracking electron transfer in redox reactions |
| Real Charge | Not a real charge, a bookkeeping tool | Represents the relative charge on an atom |
When Do They Coincide?
In some simple cases, formal charge and oxidation number might have the same numerical value, but this is coincidental and not a general rule. This coincidence usually happens in molecules with atoms of similar electronegativity. For example, in a homonuclear diatomic molecule like Cl₂, both the formal charge and oxidation number of each chlorine atom are 0.
Practical Applications:
Both formal charge and oxidation number serve different but equally important purposes in chemistry:
Formal Charge:
- Predicting Lewis Structures: Helps select the most stable Lewis structure among multiple possibilities by minimizing formal charges.
- Understanding Reactivity: Atoms with significant formal charges are more likely to participate in chemical reactions.
Oxidation Number:
- Balancing Redox Reactions: Crucial for balancing redox (reduction-oxidation) reactions, which involve electron transfer.
- Naming Compounds: Used in the nomenclature of inorganic compounds to indicate the oxidation state of an element.
- Understanding Chemical Behavior: Helps predict the behavior of elements based on their oxidation states.
Conclusion:
Formal charge and oxidation number are distinct concepts that both provide insights into electron distribution within molecules and ions. While formal charge emphasizes electron sharing in covalent bonds, ignoring electronegativity differences, oxidation number considers electron transfer based on electronegativity, providing information on relative charge and electron gain/loss. Understanding their differences and appropriate applications is essential for accurately interpreting chemical structures and reactions. They are not interchangeable; choosing the right concept depends on the specific chemical question at hand. Remember, formal charge is a bookkeeping tool, while oxidation number reflects a more realistic (though still simplified) picture of electron distribution based on the relative electronegativity of the atoms. Both are powerful tools in the chemist’s arsenal.
Latest Posts
Latest Posts
-
What Is A Subdivision In Music
Mar 19, 2025
-
Number Of Lone Pairs In H2o
Mar 19, 2025
-
How To Find The Natural Abundance Of Isotopes
Mar 19, 2025
-
Complex Number To Polar Form Converter
Mar 19, 2025
-
What Structures Are Only Found In Animal Cells
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Formal Charge The Same As Oxidation Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
