Is Half Life Represented By R
Muz Play
Mar 21, 2025 · 5 min read
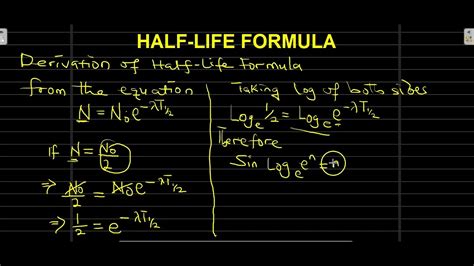
Table of Contents
Is Half-Life Represented by r? Understanding Radioactive Decay Constants
The question, "Is half-life represented by r?" requires a nuanced answer. While 'r' isn't the direct symbol for half-life (that's typically represented by t<sub>½</sub>), it's closely related to a crucial parameter in radioactive decay calculations: the decay constant, often denoted by λ (lambda) or sometimes k. This decay constant, λ, is inversely proportional to the half-life, meaning they're inextricably linked. This article will delve into the relationship between half-life, the decay constant, and how they're used to model radioactive decay. We'll explore the equations, provide examples, and clarify the common misconceptions surrounding this topic.
Understanding Radioactive Decay
Radioactive decay is a fundamental process in nuclear physics where unstable atomic nuclei lose energy by emitting radiation. This process occurs spontaneously and at a statistically predictable rate. Several types of radiation can be emitted, including alpha particles, beta particles, and gamma rays. The rate at which a radioactive substance decays is characterized by its half-life and decay constant.
The Decay Constant (λ or k)
The decay constant, λ (lambda) or sometimes k, represents the probability of a single nucleus decaying in a unit of time. A higher decay constant indicates a faster decay rate. It's crucial to understand that λ doesn't represent the number of atoms decaying, but rather the probability of decay per atom per unit time.
Mathematically, the decay constant is defined by:
dN/dt = -λN
Where:
dN/dtrepresents the rate of change in the number of radioactive atoms (N) over time (t). The negative sign indicates a decrease in the number of radioactive atoms.Nis the number of radioactive atoms at time t.λis the decay constant.
This is a first-order differential equation, which, when solved, yields the exponential decay equation:
N(t) = N₀e^(-λt)
Where:
N(t)is the number of radioactive atoms remaining after time t.N₀is the initial number of radioactive atoms at time t = 0.eis the base of the natural logarithm (approximately 2.718).λis the decay constant.tis the elapsed time.
Half-Life (t<sub>½</sub>)
The half-life (t<sub>½</sub>) is the time it takes for half of the radioactive atoms in a sample to decay. It's a crucial characteristic used to identify different radioactive isotopes and predict their decay behavior.
The relationship between half-life and the decay constant is:
t<sub>½</sub> = ln(2) / λ
or approximately:
t<sub>½</sub> ≈ 0.693 / λ
This equation shows the inverse relationship: a larger decay constant (λ) means a shorter half-life (t<sub>½</sub>), and vice versa. A substance with a large decay constant decays quickly, while a substance with a small decay constant decays slowly.
Why 'r' is Not Typically Used
While 'r' might be used in some contexts to represent a rate, it's generally not the standard symbol for the decay constant in the field of nuclear physics and radioactive decay. Using 'r' can lead to confusion, as it might be misinterpreted as a different rate constant in other scientific fields. The consistent use of λ (lambda) or k minimizes ambiguity and ensures clear communication within the scientific community.
Examples and Applications
Let's illustrate the relationship between half-life and the decay constant with a few examples:
Example 1:
Carbon-14 has a half-life of approximately 5,730 years. We can calculate its decay constant:
λ = ln(2) / t<sub>½</sub> = ln(2) / 5730 years ≈ 1.21 x 10⁻⁴ years⁻¹
This means that the probability of a single Carbon-14 atom decaying in one year is approximately 1.21 x 10⁻⁴.
Example 2:
Suppose a radioactive isotope has a decay constant of 0.05 per hour. We can calculate its half-life:
t<sub>½</sub> = ln(2) / λ = ln(2) / 0.05 hour⁻¹ ≈ 13.86 hours
This means that it takes approximately 13.86 hours for half of the atoms in a sample of this isotope to decay.
Beyond Simple Exponential Decay: Complex Scenarios
The simple exponential decay model assumes that only one decay process is occurring. In reality, many radioactive isotopes undergo multiple decay processes or have branching decay paths. In such cases, the decay equations become more complex, but the fundamental relationship between the decay constant and half-life remains valid for each individual decay process.
Applications of Half-Life and Decay Constant
The concepts of half-life and decay constant are crucial in various applications, including:
- Radioactive dating: Determining the age of artifacts and geological formations using the known half-lives of radioactive isotopes (e.g., Carbon-14 dating).
- Nuclear medicine: Using radioactive isotopes for diagnostic and therapeutic purposes, understanding their decay rates is essential for dosage and safety calculations.
- Nuclear power: Controlling nuclear reactions and managing radioactive waste requires precise knowledge of decay constants and half-lives.
- Environmental science: Monitoring and managing radioactive contamination in the environment requires understanding the decay processes of various isotopes.
- Geochronology: Dating rocks and minerals to understand geological time scales using radioactive isotopes with long half-lives.
Conclusion: Understanding the Interplay
In summary, while 'r' isn't the standard symbol for half-life, the decay constant (λ or k), which is inversely proportional to the half-life, is critical for understanding and modeling radioactive decay. The equations relating half-life and the decay constant are fundamental tools used across numerous scientific disciplines. The importance of using consistent and standardized notation (like λ for the decay constant and t<sub>½</sub> for half-life) cannot be overstated to avoid ambiguity and ensure accurate communication. Understanding this interplay is crucial for anyone working with radioactive materials or applying radioactive decay concepts in various scientific fields. The careful use of these parameters allows for accurate predictions and modelling in diverse applications, from archaeological dating to the safe handling of nuclear materials. Therefore, it's essential to grasp the core relationship between half-life and the decay constant to successfully navigate the complexities of radioactive decay.
Latest Posts
Latest Posts
-
Is Trigonal Planar Polar Or Nonpolar
Mar 28, 2025
-
What Is The Unique Property Of Water
Mar 28, 2025
-
What Is A Particle With A Negative Charge
Mar 28, 2025
-
Why Does Water Have High Heat Of Vaporization
Mar 28, 2025
-
The Type Of Microscope Used In Our Lab Is The
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Half Life Represented By R . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
