Is Trigonal Planar Polar Or Nonpolar
Muz Play
Mar 28, 2025 · 6 min read
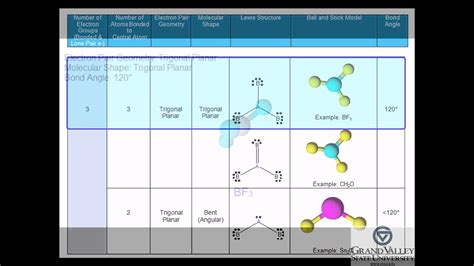
Table of Contents
Is Trigonal Planar Polar or Nonpolar? A Deep Dive into Molecular Geometry and Polarity
Determining whether a molecule is polar or nonpolar is crucial in understanding its properties and behavior. This involves considering both its molecular geometry and the polarity of its individual bonds. This article will delve into the specifics of trigonal planar molecules, exploring the factors that influence their polarity and providing clear examples to solidify understanding.
Understanding Molecular Geometry: The Trigonal Planar Shape
Before we determine polarity, let's establish a strong foundation in molecular geometry. Trigonal planar molecules exhibit a specific arrangement of atoms: a central atom surrounded by three other atoms, all lying in the same plane. The bond angles between the atoms are approximately 120 degrees. This geometry arises from the repulsion between electron pairs in the valence shell of the central atom, which strive to maximize the distance between themselves. This arrangement is commonly observed in molecules with sp² hybridization.
Key Features of Trigonal Planar Geometry:
- Central Atom: A single atom situated at the center of the molecule.
- Three Substituents: Three atoms or groups of atoms bonded to the central atom.
- Planar Structure: All four atoms lie within the same plane.
- Bond Angles: The bond angles between the substituents are approximately 120 degrees.
- Sp² Hybridization: The central atom typically exhibits sp² hybridization.
Polarity of Bonds: Electronegativity Differences
The polarity of a molecule is a direct consequence of the polarity of its individual bonds. A bond's polarity is determined by the difference in electronegativity between the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A larger electronegativity difference leads to a more polar bond, with the more electronegative atom carrying a partial negative charge (δ-) and the less electronegative atom carrying a partial positive charge (δ+).
Electronegativity and Bond Polarity:
- Nonpolar Bonds: Occur when the electronegativity difference between the atoms is negligible (typically less than 0.5). Electrons are shared relatively equally between the atoms. Examples include C-C and C-H bonds.
- Polar Bonds: Occur when there is a significant electronegativity difference between the atoms (typically greater than 0.5). Electrons are shared unequally, resulting in a dipole moment. Examples include O-H, C-O, and N-H bonds.
Determining the Polarity of Trigonal Planar Molecules: The Crucial Factor of Symmetry
The overall polarity of a trigonal planar molecule depends on both the polarity of its individual bonds and its molecular symmetry. If the three substituents surrounding the central atom are identical, the bond dipoles cancel each other out, resulting in a nonpolar molecule. However, if the substituents are different, the bond dipoles may not cancel, leading to a polar molecule.
Symmetrical Trigonal Planar Molecules (Nonpolar):
A perfectly symmetrical trigonal planar molecule has identical atoms or groups bonded to the central atom. The individual bond dipoles are equal in magnitude and oriented at 120-degree angles. These dipoles precisely cancel each other out, resulting in a net dipole moment of zero.
Examples:
- BF₃ (Boron Trifluoride): Three identical B-F bonds; the molecule is nonpolar.
- SO₃ (Sulfur Trioxide): Three identical S=O bonds; the molecule is nonpolar.
Asymmetrical Trigonal Planar Molecules (Polar):
Asymmetrical trigonal planar molecules have at least two different substituents bonded to the central atom. The bond dipoles do not cancel each other out, resulting in a net dipole moment and a polar molecule. The magnitude of the net dipole moment depends on the electronegativity differences between the substituents and their relative positions.
Examples:
- CHCl₃ (Chloroform): One C-H bond and three C-Cl bonds; the molecule is polar due to the higher electronegativity of chlorine compared to hydrogen. The C-Cl bond dipoles do not perfectly cancel the C-H bond dipole.
- COCl₂ (Phosgene): Two C-Cl bonds and one C=O bond; this molecule is also polar due to the differing electronegativities of chlorine and oxygen.
Detailed Examination of Polar Trigonal Planar Molecules
Let's analyze a polar trigonal planar molecule more closely to illustrate the concept. Consider formaldehyde (CH₂O). The carbon atom is the central atom, bonded to two hydrogen atoms and one oxygen atom.
-
Bond Polarity: The C-H bonds are relatively nonpolar, while the C=O bond is highly polar due to the significantly higher electronegativity of oxygen compared to carbon. This results in a significant dipole moment pointing from the carbon atom toward the oxygen atom.
-
Vector Addition of Dipoles: The two C-H bond dipoles have small magnitudes and partially cancel each other out. However, the much larger C=O dipole moment significantly outweighs this cancellation, resulting in a net dipole moment for the molecule. Therefore, formaldehyde is a polar molecule.
-
Consequences of Polarity: The polarity of formaldehyde affects its physical and chemical properties. For instance, it has a higher boiling point than similarly sized nonpolar molecules due to stronger intermolecular forces (dipole-dipole interactions). Its polarity also influences its reactivity and solubility in polar solvents.
Factors Affecting Polarity Beyond Basic Geometry
While the basic trigonal planar geometry provides a starting point, other factors can influence a molecule's overall polarity. These include:
- Resonance Structures: Delocalization of electrons through resonance can affect the distribution of charge within a molecule, influencing its polarity.
- Lone Pairs of Electrons: While not directly involved in bonding, lone pairs on the central atom can contribute to the overall molecular dipole moment. Their presence can significantly alter the distribution of electron density, leading to a greater degree of polarity.
- Steric Effects: The size and shape of substituents can influence bond angles and dipole orientations, impacting the overall dipole moment.
Practical Applications and Importance of Understanding Molecular Polarity
The ability to predict whether a molecule is polar or nonpolar is fundamental to various fields:
- Chemistry: Understanding polarity is crucial for predicting chemical reactivity, solubility, boiling points, and other physical properties.
- Materials Science: The polarity of molecules heavily influences the properties of materials, dictating their suitability for specific applications.
- Biology: Polarity is vital for understanding interactions between molecules in biological systems, such as protein folding and enzyme-substrate binding.
- Pharmacology: Polarity greatly affects a drug's absorption, distribution, metabolism, and excretion (ADME) properties, determining its effectiveness and safety.
Conclusion: A Comprehensive Overview
The polarity of a trigonal planar molecule hinges on its symmetry and the electronegativity differences between the atoms bonded to the central atom. Symmetrical trigonal planar molecules with identical substituents are nonpolar, while asymmetrical molecules are typically polar due to the unequal distribution of electron density. Understanding these principles is essential for predicting and explaining the diverse properties of molecules in various scientific disciplines. This knowledge forms a crucial foundation for advanced studies in chemistry, biochemistry, and materials science. Further exploration into the nuances of molecular geometry and polarity will provide deeper insight into the behavior and applications of different chemical compounds.
Latest Posts
Latest Posts
-
Which Element Is The Least Reactive
Mar 31, 2025
-
Which One Is Good Insulator Metals Metalloids Or Nonmetals
Mar 31, 2025
-
Why Are Covalent Compounds Not Conductive
Mar 31, 2025
-
What Does It Mean To Be An Artist
Mar 31, 2025
-
Reaction Of Grignard Reagent With Water
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Trigonal Planar Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
