Which One Is Good Insulator Metals Metalloids Or Nonmetals
Muz Play
Mar 31, 2025 · 5 min read
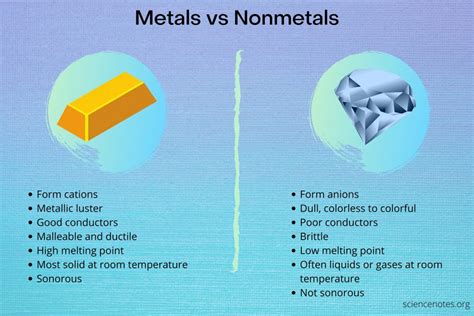
Table of Contents
Which One is a Good Insulator: Metals, Metalloids, or Nonmetals?
Understanding the insulating properties of materials is crucial in various fields, from electrical engineering to thermal management. While the terms "metal," "metalloid," and "nonmetal" broadly categorize elements based on their properties, their insulating capabilities vary significantly. This comprehensive guide will delve deep into the electronic structure and bonding characteristics that determine the insulating behavior of each group, helping you discern which one excels as an insulator.
The Fundamentals of Insulation
Before diving into the specifics of metals, metalloids, and nonmetals, let's establish a basic understanding of insulation. Insulation refers to a material's ability to resist the flow of either heat or electricity. This resistance stems from the material's atomic and molecular structure, which influences how readily electrons or phonons (vibrational energy packets) can move through it.
-
Electrical Insulation: Good electrical insulators have a large band gap, a significant energy difference between the valence band (where electrons reside in their ground state) and the conduction band (where electrons can freely move and conduct electricity). A large band gap prevents electrons from easily jumping to the conduction band, thus hindering electrical current flow.
-
Thermal Insulation: Thermal insulation, on the other hand, is related to a material's ability to impede the transfer of heat energy. This is influenced by factors like the material's density, specific heat capacity, and thermal conductivity. Materials with low thermal conductivity are effective thermal insulators because they limit the rate at which heat energy is transferred through them. Often, materials with complex molecular structures or significant air pockets are effective thermal insulators.
Metals: Excellent Conductors, Poor Insulators
Metals are characterized by their highly conductive nature, making them poor insulators. Their atomic structure plays a key role in this behavior.
Electronic Structure of Metals: A Sea of Electrons
Metallic bonding involves a "sea" of delocalized electrons. These electrons are not tightly bound to individual atoms but rather freely move throughout the metallic lattice. This mobility of electrons allows for the easy transmission of both heat and electricity. The loosely held electrons readily respond to an applied electric field, resulting in high electrical conductivity. Similarly, the free movement of electrons facilitates efficient heat transfer through the material.
Examples of Metallic Conductors:
- Copper (Cu): Widely used in electrical wiring due to its exceptional conductivity.
- Aluminum (Al): A lighter alternative to copper, also employed in electrical applications.
- Silver (Ag): Possesses the highest electrical conductivity among metals but is more expensive than copper.
- Gold (Au): Known for its excellent conductivity and resistance to corrosion, often used in electronics.
Nonmetals: The Champions of Insulation
Nonmetals, in stark contrast to metals, generally exhibit poor electrical and thermal conductivity, making them excellent insulators. Their electronic structure explains this behavior.
Covalent Bonding and Localized Electrons:
Nonmetals typically form covalent bonds, where electrons are shared between atoms. These electrons are localized within the bonds, meaning they are not free to move throughout the material. This lack of freely moving electrons drastically reduces both electrical and thermal conductivity.
Examples of Nonmetallic Insulators:
- Rubber: Widely used in electrical insulation due to its high resistivity and flexibility.
- Glass: An excellent electrical insulator and also a good thermal insulator due to its amorphous structure.
- Plastics (e.g., PVC, Teflon): A diverse range of polymers with high electrical resistance, used extensively in electrical insulation and packaging.
- Wood: A natural insulator that provides both thermal and electrical insulation.
- Ceramics (e.g., porcelain, alumina): Excellent electrical insulators, often used in high-temperature applications. Their crystalline structure and strong covalent bonds contribute to their insulating properties.
Factors Affecting Insulating Properties in Nonmetals:
The insulating properties of nonmetals can be influenced by several factors:
- Purity: Impurities in nonmetals can introduce free electrons or defects in the crystal structure, reducing their insulating capabilities.
- Temperature: At higher temperatures, some nonmetals may exhibit increased conductivity due to thermal excitation of electrons.
- Pressure: Applying high pressure can alter the interatomic distances and electronic structure, potentially affecting insulating properties.
- Moisture: Absorption of moisture can significantly reduce the insulating properties of some nonmetals.
Metalloids: A Gray Area of Conductivity
Metalloids occupy an intermediate position between metals and nonmetals, exhibiting properties of both. Their insulating behavior is more complex and less predictable compared to metals and nonmetals.
Semiconductor Behavior:
Many metalloids are semiconductors. Semiconductors have a smaller band gap than insulators, allowing for some electron mobility under specific conditions. At lower temperatures, they behave as insulators, but their conductivity increases with increasing temperature or the application of light. This makes them suitable for use in electronic devices like transistors and diodes.
Examples of Metalloids:
- Silicon (Si): The most common semiconductor, forming the basis of most integrated circuits.
- Germanium (Ge): Another important semiconductor used in some electronic components.
- Arsenic (As): Can exhibit both metallic and nonmetallic properties, depending on its bonding environment.
Conductivity Modulation in Metalloids:
The conductivity of metalloids can be significantly altered by:
- Doping: Introducing impurities into the metalloid crystal lattice to increase or decrease the number of charge carriers (electrons or holes).
- Alloying: Combining metalloids with other elements to modify their electronic structure and conductivity.
Comparing Insulation Capabilities: A Summary
| Material Type | Electrical Insulation | Thermal Insulation | Examples |
|---|---|---|---|
| Metals | Poor | Poor | Copper, Aluminum, Silver |
| Nonmetals | Excellent | Good to Excellent | Rubber, Glass, Plastics, Wood |
| Metalloids | Intermediate (Semiconductor) | Intermediate | Silicon, Germanium |
Applications of Insulators
The choice of an insulator depends heavily on the specific application and the required level of insulation. For example:
- High-voltage applications: Materials with extremely high dielectric strength, such as ceramics and certain polymers, are necessary.
- High-temperature applications: Insulators with high thermal stability, like alumina ceramics, are required.
- Flexible applications: Flexible materials like rubber and certain plastics are preferred.
- Cost-sensitive applications: Lower-cost insulators such as wood or specific plastics might be chosen.
Conclusion: Choosing the Right Insulator
The choice between metals, metalloids, and nonmetals for insulation hinges on the specific requirements of the application. Nonmetals consistently demonstrate the best insulating properties for both electricity and heat. Metalloids, with their semiconductor behavior, offer a unique set of possibilities for controlled conductivity, making them essential in electronics. Metals, due to their excellent conductivity, are fundamentally unsuitable as insulators. This detailed overview helps clarify the differences in insulating capabilities across these material classes, equipping you with the knowledge to select the most appropriate material for your specific needs. Understanding the interplay between electronic structure, bonding, and macroscopic properties is key to selecting effective insulation materials across diverse applications.
Latest Posts
Latest Posts
-
Map Of N Africa And Sw Asia
Apr 01, 2025
-
Converting Water From A Liquid To A Gas Requires
Apr 01, 2025
-
Comparison Of Somatic And Autonomic Nervous Systems
Apr 01, 2025
-
How Many Valence Electrons Do Alkaline Earth Metals Have
Apr 01, 2025
-
What Does It Mean If Keq 1
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which One Is Good Insulator Metals Metalloids Or Nonmetals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
