What Does It Mean If Keq 1
Muz Play
Apr 01, 2025 · 5 min read
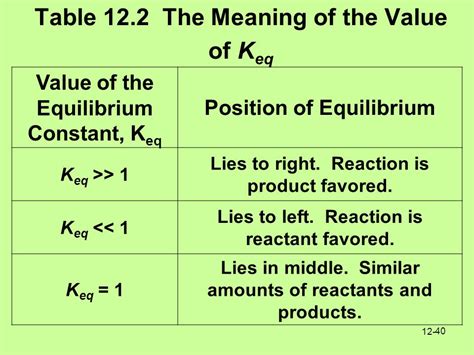
Table of Contents
What Does it Mean if Keq = 1? Understanding Equilibrium Constants
The equilibrium constant, Keq, is a fundamental concept in chemistry that describes the relationship between reactants and products at equilibrium for a reversible reaction. A reversible reaction is one that can proceed in both the forward and reverse directions. Understanding Keq, and specifically what it signifies when it equals 1, is crucial for comprehending chemical processes and predicting reaction outcomes. This article delves deep into the meaning of Keq = 1, exploring its implications and providing illustrative examples.
Equilibrium: A Dynamic State
Before we dissect the meaning of Keq = 1, let's establish a firm grasp on the concept of chemical equilibrium. Equilibrium isn't a static state where all reactions cease; instead, it's a dynamic equilibrium. This means that both the forward and reverse reactions are occurring at the same rate. While the concentrations of reactants and products remain constant at equilibrium, the individual molecules are constantly reacting and reforming. Imagine a busy highway; cars are constantly entering and exiting, but the overall traffic density remains consistent – that's analogous to chemical equilibrium.
The Equilibrium Constant (Keq): A Quantitative Measure
The equilibrium constant, Keq, provides a quantitative measure of the relative amounts of reactants and products at equilibrium. It's calculated using the concentrations (or partial pressures for gases) of the reactants and products, raised to the powers of their stoichiometric coefficients in the balanced chemical equation. For a general reaction:
aA + bB ⇌ cC + dD
The equilibrium constant expression is:
Keq = ([C]^c * [D]^d) / ([A]^a * [B]^b)
Where:
- [A], [B], [C], and [D] represent the equilibrium concentrations of reactants and products.
- a, b, c, and d are the stoichiometric coefficients from the balanced equation.
Deciphering Keq = 1: A Balanced Equilibrium
Now, let's address the core question: What does it mean if Keq = 1?
When Keq = 1, it signifies that the equilibrium concentrations of reactants and products are approximately equal. This doesn't necessarily mean they are exactly equal, but rather that the ratio of products to reactants is close to unity (1). In essence, at equilibrium, there's a nearly equal distribution of both reactants and products.
This indicates a balanced equilibrium where neither the forward nor the reverse reaction is strongly favored. The reaction has reached a state where the rates of the forward and reverse reactions are equal, resulting in a relatively equal distribution of reactants and products.
Implications of Keq = 1:
- No Strong Preference: The system doesn't show a strong preference for forming products or remaining as reactants. The reaction proceeds in both directions with comparable efficiency.
- Reversibility: A Keq of 1 clearly highlights the reversible nature of the reaction. The reaction is equally likely to proceed in either direction under the given conditions.
- Dependence on Conditions: It's crucial to remember that Keq is temperature-dependent. A Keq of 1 at one temperature might be significantly different at another temperature. Pressure can also influence Keq for gaseous reactions.
Contrasting Keq = 1 with other values:
To fully appreciate the significance of Keq = 1, let's compare it to other possible values:
- Keq >> 1 (Keq much greater than 1): This indicates that the equilibrium lies far to the right, meaning the reaction strongly favors the formation of products. The concentration of products is significantly higher than the concentration of reactants at equilibrium.
- Keq << 1 (Keq much less than 1): This signifies that the equilibrium lies far to the left, meaning the reaction strongly favors the reactants. The concentration of reactants is significantly higher than the concentration of products at equilibrium.
Examples Illustrating Keq = 1
Let's consider a few hypothetical examples to solidify our understanding:
Example 1: A Hypothetical Reaction
Consider a hypothetical reversible reaction:
A + B ⇌ C + D
If, at equilibrium, the concentrations are:
[A] = 0.5 M [B] = 0.5 M [C] = 0.5 M [D] = 0.5 M
Then, Keq = ([C] * [D]) / ([A] * [B]) = (0.5 * 0.5) / (0.5 * 0.5) = 1
This clearly shows a balanced equilibrium with nearly equal concentrations of reactants and products.
Example 2: A More Complex Scenario
Let's consider a slightly more complex reaction:
2A + B ⇌ 3C
Suppose at equilibrium:
[A] = 0.1 M [B] = 0.2 M [C] = 0.3 M
Then, Keq = ([C]^3) / ([A]^2 * [B]) = (0.3^3) / (0.1^2 * 0.2) = 13.5
In this case, Keq is significantly greater than 1, indicating that the products are favored at equilibrium. This illustrates that even with similar numerical values for concentrations, the stoichiometric coefficients play a crucial role in determining Keq.
Factors Affecting Keq: Temperature and Pressure
As mentioned earlier, Keq is not a constant under all conditions. Two major factors significantly impact its value:
1. Temperature:
Temperature significantly affects Keq, especially for reactions that are either exothermic (release heat) or endothermic (absorb heat). According to Le Chatelier's principle, increasing the temperature of an endothermic reaction shifts the equilibrium towards the products (increasing Keq), while increasing the temperature of an exothermic reaction shifts the equilibrium towards the reactants (decreasing Keq).
2. Pressure:
Pressure primarily affects the Keq of reactions involving gases. Increasing the pressure of a gaseous reaction shifts the equilibrium towards the side with fewer gas molecules. This adjustment impacts the relative concentrations of reactants and products, thus altering the Keq.
Practical Applications of Understanding Keq
The understanding of Keq and its implications holds considerable practical value across various fields:
- Chemical Engineering: Optimizing reaction conditions (temperature, pressure, reactant concentrations) to maximize product yield relies heavily on manipulating Keq.
- Environmental Chemistry: Assessing the fate and transport of pollutants in the environment requires an understanding of the equilibrium constants of various chemical reactions.
- Biochemistry: Many biochemical reactions are reversible, and understanding their equilibrium constants is crucial for comprehending metabolic pathways and enzyme kinetics.
- Materials Science: The synthesis and properties of many materials depend on the equilibrium constants of the reactions used to produce them.
Conclusion: The Significance of Keq = 1
In conclusion, a Keq of 1 signifies a balanced equilibrium where the concentrations of reactants and products are approximately equal. It highlights the reversible nature of the reaction and indicates that neither the forward nor the reverse reaction is significantly favored under the given conditions. However, it's paramount to remember that Keq is temperature and pressure dependent, and its value can change significantly with alterations in these parameters. A thorough understanding of Keq is crucial for predicting reaction outcomes, optimizing reaction conditions, and interpreting the behavior of chemical systems across diverse fields of study. It is a fundamental concept with far-reaching practical implications.
Latest Posts
Latest Posts
-
2 Protons 2 Neutrons 2 Electrons
Apr 02, 2025
-
Is Channel Protein Active Or Passive
Apr 02, 2025
-
Is Aluminum A Metal Or Metalloid
Apr 02, 2025
-
Which Of These Occurs During Reproduction Choose 1 Answer
Apr 02, 2025
-
The Atrioventricular Av Valves Are Closed
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Does It Mean If Keq 1 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
