How Many Valence Electrons Do Alkaline Earth Metals Have
Muz Play
Apr 01, 2025 · 6 min read
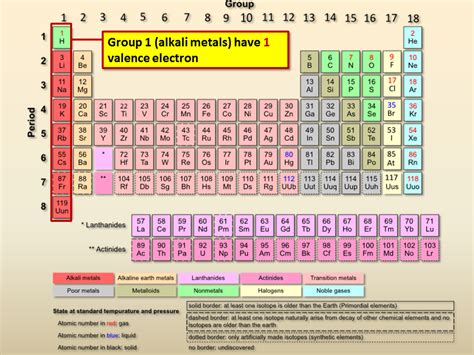
Table of Contents
How Many Valence Electrons Do Alkaline Earth Metals Have? A Deep Dive into Group 2 Elements
Alkaline earth metals, the stars of Group 2 on the periodic table, are known for their reactivity and unique properties. Understanding their electron configuration is key to unlocking the secrets behind their behavior. This comprehensive guide delves deep into the question: how many valence electrons do alkaline earth metals have? We'll explore the concept of valence electrons, examine the electron configuration of each alkaline earth metal, and discuss how this electron count influences their chemical properties and reactivity.
Understanding Valence Electrons: The Key to Reactivity
Before diving into the specifics of alkaline earth metals, let's establish a firm grasp on the concept of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. These electrons are the primary players in chemical bonding, determining how an atom will interact with other atoms to form molecules or compounds. Atoms strive for stability, often achieved by having a full outermost shell (usually eight electrons, following the octet rule). This drive for stability is the fundamental force behind chemical reactions.
The number of valence electrons an atom possesses directly impacts its reactivity. Atoms with nearly full valence shells tend to gain electrons to achieve stability, while those with few valence electrons tend to lose them. This electron transfer forms ions and drives the formation of ionic compounds. Atoms with half-filled valence shells may share electrons, forming covalent bonds.
Alkaline Earth Metals: A Closer Look at Group 2
The alkaline earth metals comprise a group of six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They are all characterized by having two valence electrons. This consistent number of valence electrons is what defines them as a group and explains their shared chemical characteristics.
Let's examine the electron configuration of each alkaline earth metal to illustrate this point:
- Beryllium (Be): 1s² 2s² - Two electrons in the outermost shell (n=2).
- Magnesium (Mg): 1s² 2s² 2p⁶ 3s² - Two electrons in the outermost shell (n=3).
- Calcium (Ca): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² - Two electrons in the outermost shell (n=4).
- Strontium (Sr): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² - Two electrons in the outermost shell (n=5).
- Barium (Ba): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² - Two electrons in the outermost shell (n=6).
- Radium (Ra): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p⁶ 7s² - Two electrons in the outermost shell (n=7).
As you can see from these electron configurations, each alkaline earth metal possesses two electrons in its outermost s-orbital. This consistent configuration is responsible for their similar chemical behavior.
The Impact of Two Valence Electrons: Reactivity and Properties
The presence of two valence electrons profoundly influences the chemical properties and reactivity of alkaline earth metals. Because achieving a stable octet requires losing two electrons, alkaline earth metals readily ionize, forming divalent cations with a +2 charge (e.g., Mg²⁺, Ca²⁺). This ionization is easier than for Group 1 elements (alkali metals) which have only one valence electron to lose and therefore exhibit lower ionization energies.
This tendency to lose two electrons leads to several key characteristics:
-
Reactivity: Alkaline earth metals are highly reactive, though less so than the alkali metals. Their reactivity increases down the group, due to increasing atomic radius and decreasing ionization energy. This means that barium and radium are more reactive than beryllium and magnesium.
-
Formation of Ionic Compounds: The +2 ions formed by alkaline earth metals readily combine with anions (negatively charged ions) to form ionic compounds. For example, calcium reacts with oxygen to form calcium oxide (CaO), a common component of cement.
-
Metallic Bonding: Alkaline earth metals exhibit strong metallic bonding due to the relatively free movement of their valence electrons within the metallic lattice. This results in properties like high electrical and thermal conductivity. They are also malleable and ductile.
-
Oxidation: Because they readily lose electrons, alkaline earth metals are easily oxidized (lose electrons). They react readily with air (oxygen) forming oxides and with water forming hydroxides and hydrogen gas. Beryllium is an exception - it is relatively unreactive towards water due to its high ionization energy and forms a protective oxide layer.
Specific Examples: Illustrating Reactivity
Let's examine a few specific examples illustrating the reactivity of alkaline earth metals:
-
Magnesium's Reaction with Oxygen: Magnesium burns brightly in air, producing a dazzling white light. This is due to the rapid oxidation of magnesium, forming magnesium oxide (MgO).
-
Calcium's Reaction with Water: Calcium reacts more vigorously with water than magnesium, producing calcium hydroxide (Ca(OH)₂), and hydrogen gas (H₂).
-
Beryllium's Inertness: While generally considered a reactive element, beryllium's tendency to form a protective oxide layer upon exposure to air contributes to its comparatively low reactivity compared to other group 2 elements.
Exceptions and Anomalies: Understanding Beryllium's Unique Behavior
While all alkaline earth metals share the characteristic of two valence electrons, beryllium stands out as an exception to some general trends. Its smaller atomic size and higher ionization energy result in some differences:
-
Lower Reactivity: Beryllium is significantly less reactive than the other alkaline earth metals. It reacts slowly with water and acids. This reduced reactivity is attributed to its high ionization energy and the strong polarization of its bonds.
-
Amphoteric Oxide: Beryllium oxide (BeO) is amphoteric, meaning it can react with both acids and bases. This contrasts with the typically basic oxides of the other alkaline earth metals.
-
Covalent Character: Due to its smaller size and high charge density, beryllium often forms covalent bonds rather than purely ionic ones, unlike the heavier alkaline earth metals which largely exhibit ionic bonding.
Conclusion: The Significance of Two Valence Electrons
In conclusion, the answer to the question, "How many valence electrons do alkaline earth metals have?" is definitively two. This fundamental characteristic dictates their chemical properties, reactivity, and the types of compounds they form. Understanding the implications of this electron configuration is crucial for comprehending their behavior in various chemical reactions and applications across diverse fields, including materials science, metallurgy, and chemical engineering. While beryllium exhibits some unique properties due to its smaller size, the consistent presence of two valence electrons unites the alkaline earth metals as a distinct and fascinating group on the periodic table. Their predictable reactivity makes them valuable elements in various industrial processes and applications.
Latest Posts
Latest Posts
-
What Is The Unit Of Inheritance
Apr 02, 2025
-
Angular Momentum Operator In Spherical Coordinates
Apr 02, 2025
-
Rules For Writing A Chemical Formula
Apr 02, 2025
-
Iron Rusting Physical Or Chemical Change
Apr 02, 2025
-
2 Protons 2 Neutrons 2 Electrons
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Do Alkaline Earth Metals Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
