Why Are Covalent Compounds Not Conductive
Muz Play
Mar 31, 2025 · 5 min read
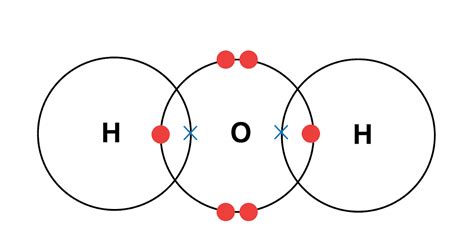
Table of Contents
Why Are Covalent Compounds Not Conductive?
Covalent compounds, formed by the sharing of electrons between atoms, are notoriously poor conductors of electricity. This stark contrast to the conductive properties of ionic and metallic compounds stems from the fundamental differences in their bonding and electron structure. Understanding this difference is key to grasping the behaviour of various materials in electrical circuits and applications. This article delves deep into the reasons behind the non-conductivity of covalent compounds, exploring the nuances of their bonding, electron mobility, and exceptions to this general rule.
The Nature of Covalent Bonding
At the heart of understanding the non-conductive nature of covalent compounds lies the understanding of covalent bonding itself. Unlike ionic compounds, where electrons are transferred from one atom to another, creating charged ions, covalent bonding involves the sharing of electrons between atoms. This sharing creates a stable molecule where the atoms achieve a full outer electron shell, satisfying the octet rule (or duet rule for hydrogen).
Shared Electron Pairs and Localized Electrons
These shared electrons are held tightly between the bonded atoms, forming a localized electron cloud. This means the electrons are not free to move independently throughout the material. They are constrained within the confines of the individual molecule. This localization is the primary reason for the poor conductivity of most covalent compounds.
Strong Intramolecular Bonds, Weak Intermolecular Forces
While the bonds within a covalent molecule are strong, the forces between molecules are generally weak. These intermolecular forces, such as van der Waals forces, hydrogen bonds, and dipole-dipole interactions, are significantly weaker than the covalent bonds themselves. This weak intermolecular interaction further restricts electron mobility. The electrons remain confined within their respective molecules, unable to traverse the gaps between them to carry an electric current.
Contrast with Ionic and Metallic Conductors
Let's contrast this with ionic and metallic compounds to highlight the difference.
Ionic Compounds: Conductivity in the Molten State
Ionic compounds are formed by the electrostatic attraction between oppositely charged ions. When dissolved in water or molten (melted), these ions become mobile and can carry an electric current. The free movement of these charged particles facilitates the flow of electricity. In the solid state, however, the ions are held rigidly in a lattice structure, preventing conductivity.
Metallic Compounds: The Electron Sea Model
Metallic compounds exhibit excellent conductivity due to the "sea" of delocalized electrons surrounding positively charged metal ions. These electrons are not bound to any specific atom and are free to move throughout the entire metal structure. This free movement of electrons allows for the easy flow of electric current.
Why Covalent Compounds Don't Conduct (Except in Specific Cases)
The inability of most covalent compounds to conduct electricity directly stems from the lack of freely moving charged particles. There are no free ions (like in ionic compounds) or delocalized electrons (like in metals). The electrons are tightly bound within the covalent bonds, limiting their mobility and preventing the flow of electric current.
The Role of Electron Mobility
Electrical conductivity is directly related to electron mobility. Materials with high electron mobility are good conductors because electrons can easily move in response to an applied electric field. Covalent compounds, by their nature, have low electron mobility, leading to their poor conductivity.
Exceptions to the Rule: Conductivity in Specific Covalent Compounds
While the majority of covalent compounds are non-conductive, some exceptions exist under specific conditions. These exceptions don't contradict the fundamental principles discussed earlier but highlight the complexities of material behaviour.
Semi-Conductors: Silicon and Germanium
Semi-conductors, such as silicon and germanium, are covalent compounds with unique electrical properties. While they are poor conductors at low temperatures, their conductivity increases significantly with increasing temperature or the addition of impurities (doping). This increase in conductivity is due to the promotion of electrons from the valence band to the conduction band, creating charge carriers. Doping introduces additional electrons or "holes" (absence of electrons) into the structure, further enhancing conductivity. This makes them essential components in electronics.
Graphite: A Special Case of Covalent Bonding
Graphite, an allotrope of carbon, is a notable exception. While carbon atoms within individual layers are bonded covalently, the layers are held together by weak van der Waals forces. The electrons in the delocalized pi bonds within each layer are relatively mobile, allowing for conductivity along the plane of the layers. This explains graphite's use as a component in batteries and lubricants.
Factors Influencing Conductivity in Covalent Compounds
Several factors can influence the conductivity (or lack thereof) in covalent compounds:
- Bond Polarity: While not directly causing conductivity, the presence of polar covalent bonds can influence the material's response to an electric field. Polar molecules may exhibit some degree of dielectric polarization, but this doesn't result in significant electrical conduction.
- Impurities: The presence of impurities in covalent compounds can affect conductivity. These impurities might introduce charge carriers, increasing conductivity, similar to the doping effect in semiconductors.
- Temperature: Temperature plays a significant role in the conductivity of some covalent compounds, particularly semiconductors. Increasing temperature provides more energy for electrons to overcome the energy gap and move to the conduction band.
- Pressure: Applying pressure to a covalent material can alter the interatomic distances and affect electron mobility. In some cases, this can lead to a change in conductivity.
Applications Based on Non-Conductivity
The non-conductive nature of many covalent compounds is exploited in numerous applications. Many plastics and polymers, which are largely covalent, are used as insulators in electrical equipment to prevent short circuits and ensure safety. These materials are critical components in various electronic devices and power systems.
Conclusion
In conclusion, the non-conductive nature of covalent compounds is primarily attributed to the localized nature of electrons within the covalent bonds. The absence of free ions or delocalized electrons prevents the flow of electricity. While some exceptions exist, like semi-conductors and graphite, these exceptions highlight unique electronic structures and conditions that deviate from the typical behaviour of covalent compounds. Understanding this fundamental difference in bonding and electron behaviour is vital for selecting materials with the appropriate electrical properties for diverse applications in various fields of science and technology. The non-conductive nature of many covalent compounds is not a drawback but a crucial property exploited in countless applications.
Latest Posts
Latest Posts
-
Converting Water From A Liquid To A Gas Requires
Apr 01, 2025
-
Comparison Of Somatic And Autonomic Nervous Systems
Apr 01, 2025
-
How Many Valence Electrons Do Alkaline Earth Metals Have
Apr 01, 2025
-
What Does It Mean If Keq 1
Apr 01, 2025
-
What Is The Chemical Composition Of Amethyst
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Are Covalent Compounds Not Conductive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
