Which Element Is The Least Reactive
Muz Play
Mar 31, 2025 · 5 min read
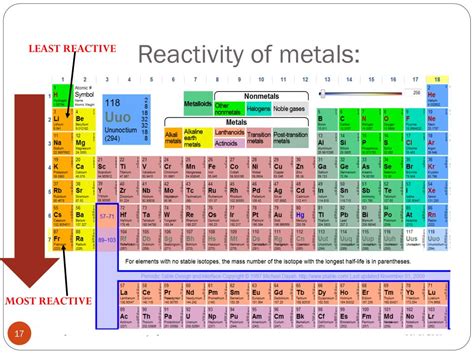
Table of Contents
Which Element is the Least Reactive? Unveiling the Chemical Inertness of Noble Gases
The periodic table, a beautifully organized chart of the elements, reveals much about the behavior of matter. One of the most striking features is the clear distinction in reactivity between different groups of elements. While some elements readily engage in chemical reactions, others stubbornly resist them. This article delves into the fascinating world of chemical reactivity, focusing on the element group famously known for their extreme inertness: the noble gases. We'll explore why they hold this title, examining their electronic configurations, historical discoveries, and unique properties that make them the least reactive elements.
Understanding Chemical Reactivity
Before identifying the least reactive element, it's crucial to understand what drives chemical reactivity. At the heart of it lies the quest for electronic stability. Atoms are inherently unstable when their outermost electron shell (valence shell) isn't full. They strive to achieve a stable electron configuration, typically resembling that of a noble gas, by gaining, losing, or sharing electrons. This drive toward stability dictates their chemical behavior.
Highly reactive elements, like alkali metals (Group 1) and halogens (Group 17), readily react because they are only one electron away from achieving a stable noble gas configuration. Alkali metals readily lose one electron, while halogens readily gain one electron. This electron transfer results in the formation of ionic compounds and releases significant energy.
The Noble Gases: A Family of Inert Elements
The noble gases, also known as inert gases, occupy Group 18 of the periodic table. This group includes helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and the synthetically created oganesson (Og). These elements are characterized by their exceptional resistance to chemical reactions, a property stemming directly from their electronic structure.
The Octet Rule and Electronic Configuration
The key to understanding the noble gases' inertness lies in the octet rule. This rule states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell containing eight electrons (except for helium, which requires only two electrons for a full shell). Noble gases naturally possess this stable electron configuration. Their valence shells are completely filled, meaning they have no strong tendency to gain, lose, or share electrons to form chemical bonds.
Helium (He): With two electrons filling its only electron shell, helium perfectly exemplifies this stable configuration.
Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn), and Oganesson (Og): These elements all possess eight electrons in their outermost shell, satisfying the octet rule and contributing to their inertness.
The Exception: Xenon's Unusual Reactivity
While noble gases are renowned for their inertness, xenon stands out as a slight exception. Under specific conditions, such as exposure to highly reactive fluorine, xenon can form compounds. This is because xenon, being the largest of the naturally occurring noble gases, has a relatively weak hold on its outer electrons. This makes it slightly more susceptible to chemical interaction compared to lighter noble gases. However, even xenon's reactivity is significantly lower than that of most other elements.
Historical Discoveries and Their Impact
The discovery of noble gases significantly impacted our understanding of chemistry. Their unique properties challenged existing theories and expanded the boundaries of chemical knowledge. The inert nature of these elements led to their use in various applications where their non-reactivity was paramount.
Early Discoveries and the Challenge to Existing Theories
The discovery of argon in 1894 by Lord Rayleigh and Sir William Ramsay marked a turning point. Its unexpected inertness didn't fit the then-existing periodic classification. The subsequent discovery of other noble gases further solidified the need to revise the periodic table and develop a more comprehensive understanding of chemical bonding.
Applications Leveraging Inertness
The inertness of noble gases has led to their widespread use in diverse applications:
- Helium (He): Used in balloons, airships, and cryogenics due to its low density and boiling point.
- Neon (Ne): Famous for its vibrant red glow in neon signs, it’s also utilized in lasers.
- Argon (Ar): Used as an inert atmosphere in welding and other industrial processes to prevent oxidation.
- Krypton (Kr): Used in high-intensity lighting and lasers.
- Xenon (Xe): Used in medical imaging (CT scans) and high-intensity lighting.
- Radon (Rn): Though radioactive, its inert nature prevents it from easily bonding and spreading throughout the environment in more dangerous ways.
Comparing Reactivity: Noble Gases vs. Other Elements
To fully appreciate the exceptional inertness of noble gases, it's helpful to compare them with other elements. Alkali metals, for instance, react violently with water, while halogens readily react with many metals. Noble gases, however, show virtually no reactivity under ordinary conditions. This extreme difference highlights their unique position in the periodic table.
Factors Affecting Noble Gas Reactivity (Though Minimal)
While the noble gases are generally considered non-reactive, some factors can influence their behavior, albeit minimally:
- Size: Larger noble gas atoms (like xenon) have a weaker hold on their valence electrons, making them slightly more reactive.
- Pressure and Temperature: Extreme conditions of high pressure and low temperature can induce some reactivity in noble gases.
- High-Energy Radiation: Under specific irradiation, some reactions of noble gases with other reactive species may take place.
Conclusion: The Unchallenged Reign of Inertness
The noble gases, particularly helium and neon, maintain their unchallenged position as the least reactive elements. Their stable electronic configuration, dictated by the filled valence shells, is the primary reason for their extreme inertness. Although xenon demonstrates some reactivity under very specific conditions, its overall reactivity remains far lower than that of other elements on the periodic table. The discovery and study of these elements have profoundly impacted our understanding of chemistry, highlighting the central role of electronic structure in determining chemical behavior. Their unique properties have found numerous applications in various fields, emphasizing their importance in modern science and technology. While future research may reveal further nuances in their reactivity, the noble gases continue to stand out as a testament to the remarkable order and predictability inherent within the periodic table.
Latest Posts
Latest Posts
-
Northern Blot Southern Blot Western Blot
Apr 01, 2025
-
Map Of N Africa And Sw Asia
Apr 01, 2025
-
Converting Water From A Liquid To A Gas Requires
Apr 01, 2025
-
Comparison Of Somatic And Autonomic Nervous Systems
Apr 01, 2025
-
How Many Valence Electrons Do Alkaline Earth Metals Have
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Element Is The Least Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
