Is Hc2h3o2 A Strong Or Weak Acid
Muz Play
Mar 19, 2025 · 6 min read
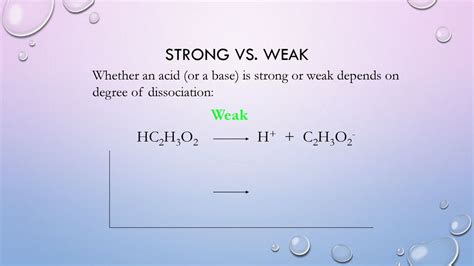
Table of Contents
Is HC₂H₃O₂ a Strong or Weak Acid? A Comprehensive Guide
Acetic acid, with the chemical formula HC₂H₃O₂, is a common household chemical found in vinegar. But beyond its culinary applications, understanding its acidic nature is crucial in various scientific and industrial contexts. This comprehensive guide will delve deep into the question: Is HC₂H₃O₂ a strong or weak acid? We'll explore the concept of acid strength, examine the properties of acetic acid, and explain why it's categorized as a weak acid.
Understanding Acid Strength: A Foundation
Before classifying acetic acid, we need to understand the concept of acid strength. Acid strength refers to the ability of an acid to donate a proton (H⁺) in a solution. This proton donation leads to the formation of hydronium ions (H₃O⁺), which increase the acidity of the solution and lower its pH.
Strong acids readily and completely dissociate in water, meaning almost all of their molecules donate a proton. This results in a high concentration of H₃O⁺ ions and a low pH. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃).
Conversely, weak acids only partially dissociate in water. A significant portion of their molecules remain undissociated, resulting in a lower concentration of H₃O⁺ ions and a higher pH compared to strong acids. The extent of dissociation is quantified by the acid dissociation constant, Ka.
Acetic Acid: A Closer Look
Acetic acid, also known as ethanoic acid, is an organic compound with the chemical formula HC₂H₃O₂. Its structure consists of a methyl group (CH₃) attached to a carboxyl group (-COOH). The carboxyl group is responsible for the acidic nature of acetic acid. The hydrogen atom in the carboxyl group is relatively easily donated as a proton.
However, the donation isn't complete. This is the key to understanding why acetic acid is a weak acid.
The Evidence: Why HC₂H₃O₂ is a Weak Acid
Several lines of evidence strongly support the classification of HC₂H₃O₂ as a weak acid:
1. Low Acid Dissociation Constant (Ka)
The acid dissociation constant (Ka) is a quantitative measure of acid strength. A higher Ka value indicates a stronger acid, while a lower Ka value indicates a weaker acid. For acetic acid, the Ka value is approximately 1.8 x 10⁻⁵ at 25°C. This extremely small Ka value is a definitive indicator of its weak acidic nature. Strong acids have Ka values much closer to 1 or even greater.
2. Partial Dissociation in Water
When acetic acid is dissolved in water, only a small fraction of its molecules dissociate into acetate ions (C₂H₃O₂⁻) and hydronium ions (H₃O⁺). The majority of the acetic acid molecules remain undissociated. This incomplete dissociation is a hallmark of weak acids. The equilibrium reaction can be represented as follows:
HC₂H₃O₂(aq) + H₂O(l) ⇌ H₃O⁺(aq) + C₂H₃O₂⁻(aq)
The double arrow (⇌) signifies the equilibrium between the reactants (acetic acid and water) and the products (hydronium ions and acetate ions). The equilibrium lies significantly to the left, indicating that most of the acetic acid remains undissociated.
3. pH of Acetic Acid Solutions
The pH of a solution is a measure of its acidity or alkalinity. A lower pH indicates a higher acidity. A solution of acetic acid with a moderate concentration will have a pH significantly higher than that of a strong acid of the same concentration. This higher pH is a direct consequence of the lower concentration of H₃O⁺ ions resulting from the incomplete dissociation of acetic acid.
4. Conductivity
Strong acids are excellent conductors of electricity because they readily dissociate into ions in solution. Weak acids, on the other hand, are poor conductors of electricity due to their low degree of dissociation. Acetic acid solutions exhibit low electrical conductivity, further supporting its classification as a weak acid.
5. Titration Curves
Titration curves, obtained by plotting the pH of a solution against the volume of added base, can be used to determine the strength of an acid. The titration curve of a weak acid like acetic acid shows a gradual change in pH around the equivalence point, unlike the sharp change observed for strong acids. This gradual pH change during titration is another characteristic feature distinguishing weak acids from their stronger counterparts.
Practical Implications of Acetic Acid's Weakness
The weak acidic nature of HC₂H₃O₂ has several important practical implications:
-
Vinegar's Mild Acidity: The mild acidity of vinegar, which is primarily a dilute solution of acetic acid, is a consequence of its weak acidic nature. It's safe for use in food preparation and cleaning, although precautions should still be taken as it is still an acid.
-
Buffer Solutions: Acetic acid, along with its conjugate base, acetate (C₂H₃O₂⁻), can be used to prepare buffer solutions. Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. This property is crucial in many biological and chemical systems.
-
Industrial Applications: Acetic acid is used in a wide variety of industrial applications, including the production of plastics, textiles, and pharmaceuticals. Its weak acidity is a factor considered in these applications, as it allows for controlled reactions without causing excessive damage or unwanted side reactions.
-
Chemical Synthesis: Acetic acid plays an important role as a reactant or catalyst in many chemical syntheses. Its relatively weak nature allows for greater control and selectivity in these reactions compared to stronger acids.
Distinguishing Weak from Strong: A Summary Table
| Feature | Strong Acid (e.g., HCl) | Weak Acid (e.g., HC₂H₃O₂) |
|---|---|---|
| Dissociation | Complete | Partial |
| Ka Value | High (close to or > 1) | Low (much less than 1) |
| H₃O⁺ Concentration | High | Low |
| pH | Low | High |
| Conductivity | High | Low |
| Titration Curve | Sharp pH change at equivalence point | Gradual pH change at equivalence point |
Conclusion
In conclusion, abundant evidence confirms that HC₂H₃O₂ (acetic acid) is a weak acid. Its low Ka value, partial dissociation in water, high pH of solutions, low conductivity, and characteristic titration curve all point towards its weak acidic nature. This understanding is crucial in various applications, from appreciating the mild acidity of vinegar to utilizing it in buffer solutions and industrial processes. The inherent properties of acetic acid's weak acidity make it a versatile compound with broad applications in numerous fields.
Latest Posts
Latest Posts
-
How To Find First Term Of Arithmetic Sequence
Mar 19, 2025
-
Nursing Interventions For Patients With Schizophrenia
Mar 19, 2025
-
Three Important Parts Of Microscope Care
Mar 19, 2025
-
Lock And Key Model Of Enzyme Action
Mar 19, 2025
-
Integration Of Odd And Even Functions
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Hc2h3o2 A Strong Or Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
