Is O Or S More Electronegative
Muz Play
Mar 15, 2025 · 5 min read
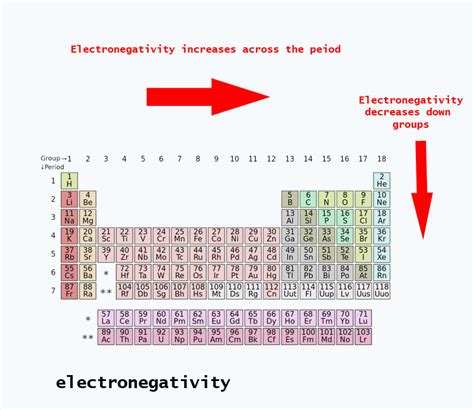
Table of Contents
Is O or S More Electronegative? A Deep Dive into Electronegativity and Periodic Trends
Electronegativity, a fundamental concept in chemistry, dictates how strongly an atom attracts electrons within a chemical bond. Understanding electronegativity differences is crucial for predicting bond polarity, molecular geometry, and overall chemical reactivity. This in-depth article will explore the electronegativity of oxygen (O) and sulfur (S), comparing their values and delving into the underlying reasons for the observed differences. We'll also examine the broader context of electronegativity trends within the periodic table and how these trends relate to various chemical properties.
Understanding Electronegativity
Electronegativity isn't a directly measurable quantity like mass or charge. Instead, it's a relative property, reflecting an atom's tendency to attract shared electrons in a covalent bond. Several scales exist to quantify electronegativity, the most commonly used being the Pauling scale. On the Pauling scale, fluorine (F) is assigned the highest electronegativity value of 4.0, with other elements assigned values relative to fluorine. Higher values indicate a stronger attraction for electrons.
Key Factors Influencing Electronegativity:
- Nuclear Charge: A greater number of protons in the nucleus leads to a stronger pull on electrons.
- Atomic Radius: Smaller atoms have electrons closer to the nucleus, experiencing a stronger attractive force.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge felt by the valence electrons.
Comparing Oxygen (O) and Sulfur (S)
Oxygen and sulfur both belong to Group 16 (or VIA) of the periodic table, also known as the chalcogens. They share similar chemical properties due to their identical valence electron configuration (ns²np⁴), but their electronegativity values differ significantly.
Electronegativity Values (Pauling Scale):
- Oxygen (O): Approximately 3.44
- Sulfur (S): Approximately 2.58
Clearly, oxygen is significantly more electronegative than sulfur. This difference can be explained by examining the factors mentioned earlier:
1. Atomic Radius
Oxygen has a considerably smaller atomic radius than sulfur. This means its valence electrons are much closer to the positively charged nucleus, experiencing a stronger electrostatic attraction. The increased proximity overcomes the effect of increased shielding in sulfur.
2. Shielding Effect
While both oxygen and sulfur have inner electrons shielding the valence electrons, the increased number of electron shells in sulfur leads to greater shielding. This reduces the effective nuclear charge experienced by the valence electrons in sulfur compared to oxygen. The weaker effective nuclear charge contributes to sulfur's lower electronegativity.
3. Nuclear Charge
While sulfur has a higher nuclear charge than oxygen (16 protons vs. 8 protons), this increase is counteracted by the significantly larger atomic radius and increased shielding effect. The combined effects of atomic radius and shielding are more influential than the increase in nuclear charge in determining the electronegativity difference.
Implications of Electronegativity Differences
The difference in electronegativity between oxygen and sulfur has significant implications for their chemical behavior:
-
Bond Polarity: Bonds between oxygen and other less electronegative atoms (like hydrogen or carbon) are significantly more polar than bonds between sulfur and the same atoms. This results in molecules containing oxygen often exhibiting greater dipole moments. For instance, water (H₂O) is a highly polar molecule, while hydrogen sulfide (H₂S) is much less polar.
-
Reactivity: Oxygen's higher electronegativity makes it a much more potent oxidizing agent than sulfur. Oxygen readily accepts electrons to achieve a stable octet configuration, leading to its high reactivity in many chemical reactions, including combustion. Sulfur's lower electronegativity makes it a less aggressive oxidizing agent, although it still participates in oxidation-reduction reactions.
-
Acidity: The difference in electronegativity affects the acidity of their respective hydrides (water and hydrogen sulfide). The highly polar O-H bonds in water make it a weaker acid than the less polar S-H bonds in hydrogen sulfide.
Electronegativity Trends in the Periodic Table
Understanding the electronegativity of oxygen and sulfur requires considering the broader trends within the periodic table:
-
Across a Period: Electronegativity generally increases as you move from left to right across a period. This is because the nuclear charge increases while the atomic radius remains relatively constant, leading to a stronger attraction for electrons.
-
Down a Group: Electronegativity generally decreases as you move down a group. The increasing atomic radius and increased shielding effect outweigh the increase in nuclear charge, resulting in weaker attraction for electrons.
The trends accurately reflect the relative electronegativities of oxygen and sulfur. Moving down Group 16, sulfur experiences a significant decrease in electronegativity compared to oxygen.
Beyond the Pauling Scale: Other Electronegativity Scales
While the Pauling scale is widely used, other electronegativity scales exist, including the Mulliken scale and the Allred-Rochow scale. These scales employ different approaches to quantify electronegativity, but they all generally show the same trend: oxygen is more electronegative than sulfur.
The Mulliken scale, for example, relates electronegativity to ionization energy and electron affinity. Oxygen's higher ionization energy (energy required to remove an electron) and electron affinity (energy released when an electron is added) contribute to its higher Mulliken electronegativity compared to sulfur.
Conclusion: Oxygen Reigns Supreme in Electronegativity
In summary, oxygen (O) is definitively more electronegative than sulfur (S). This difference arises primarily from oxygen's smaller atomic radius and reduced shielding effect, which allow for a stronger attraction to electrons in a chemical bond. This electronegativity difference significantly impacts the chemical properties of oxygen and sulfur-containing compounds, influencing bond polarity, reactivity, and acidity. Understanding these electronegativity trends is essential for comprehending a wide range of chemical phenomena and predicting the behavior of molecules. The consistent observation across different electronegativity scales reinforces the validity of this fundamental chemical principle. The difference in electronegativity between oxygen and sulfur highlights the importance of considering atomic structure and periodic trends in understanding chemical reactivity and bonding.
Latest Posts
Latest Posts
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
-
Evidence Of Light As A Particle
Mar 17, 2025
-
Do Achiral Molecules Have A Plane Of Symmetry
Mar 17, 2025
-
Acto 3 Escena 1 Romeo Y Julieta
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is O Or S More Electronegative . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
