Is Oxygen A Metal Or Nonmetal Or Metalloid
Muz Play
Mar 24, 2025 · 5 min read
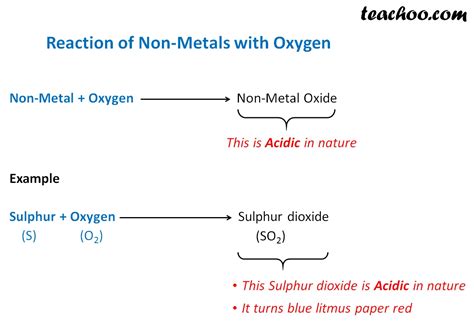
Table of Contents
Is Oxygen a Metal, Nonmetal, or Metalloid? A Deep Dive into Oxygen's Properties
Oxygen, a life-sustaining element crucial for respiration in most living organisms, often sparks curiosity about its fundamental nature. Many wonder: is oxygen a metal, nonmetal, or metalloid? This comprehensive article will explore oxygen's properties, definitively classifying it and explaining why it falls into its specific category within the periodic table. We will delve deep into its atomic structure, chemical behavior, and physical characteristics to solidify our understanding.
Understanding the Classification of Elements
Before classifying oxygen, let's establish the criteria for categorizing elements as metals, nonmetals, or metalloids. These classifications are based on several key properties:
-
Metals: Typically exhibit high electrical and thermal conductivity, malleability (ability to be hammered into thin sheets), ductility (ability to be drawn into wires), and a metallic luster (shiny appearance). They tend to lose electrons easily, forming positive ions (cations).
-
Nonmetals: Generally poor conductors of electricity and heat, brittle, lack metallic luster, and tend to gain electrons, forming negative ions (anions).
-
Metalloids (Semimetals): Possess properties intermediate between metals and nonmetals. Their conductivity can vary depending on conditions, and they often exhibit characteristics of both groups.
Oxygen's Atomic Structure and Electron Configuration
Oxygen's atomic number is 8, meaning it has 8 protons and 8 electrons in a neutral atom. Its electron configuration is 1s²2s²2p⁴. This configuration is crucial in understanding its chemical behavior. The outermost shell (valence shell) contains 6 electrons, meaning it needs to gain two electrons to achieve a stable octet (eight electrons in the valence shell), a configuration similar to the noble gas neon. This tendency to gain electrons is a hallmark of nonmetals.
Analyzing Oxygen's Physical Properties
Let's examine oxygen's physical properties to further solidify its classification:
-
State at Room Temperature: Oxygen exists as a diatomic gas (O₂) at room temperature and standard pressure. This gaseous state is characteristic of many nonmetals.
-
Conductivity: Oxygen is a poor conductor of electricity and heat. This contrasts sharply with the high conductivity exhibited by metals.
-
Appearance: Oxygen is colorless, odorless, and tasteless in its gaseous form. While some nonmetals have distinctive colors or odors, oxygen's lack thereof further supports its nonmetallic nature.
-
Malleability and Ductility: Oxygen, in its gaseous state, lacks the malleability and ductility seen in metals. It cannot be hammered into sheets or drawn into wires. Its solid form (which only exists at extremely low temperatures) is also brittle and lacks these properties.
-
Hardness: Oxygen's solid form is not particularly hard; in fact, it is quite brittle. This differs significantly from the generally high hardness of many metals.
Oxygen's Chemical Behavior: A Nonmetal's Actions
Oxygen's chemical behavior further reinforces its classification as a nonmetal. Its high electronegativity (a measure of an atom's ability to attract electrons in a chemical bond) indicates its strong tendency to gain electrons. This results in the formation of oxide ions (O²⁻) when reacting with metals or other elements.
Oxidation and Reduction Reactions: A Deeper Look
Oxygen's participation in oxidation-reduction (redox) reactions is a testament to its nonmetallic nature. In these reactions, oxygen acts as a powerful oxidizing agent, readily accepting electrons from other substances. This process leads to the formation of oxides, a hallmark of oxygen's reactivity and its role in combustion.
Consider the example of the combustion of methane (CH₄):
CH₄ + 2O₂ → CO₂ + 2H₂O
In this reaction, oxygen accepts electrons from methane, resulting in the oxidation of methane to carbon dioxide and water. Oxygen itself is reduced (gains electrons), demonstrating its strong affinity for electrons, a characteristic of nonmetals.
Comparing Oxygen to Metals and Metalloids
Comparing oxygen's properties with those of metals and metalloids strengthens the case for its nonmetallic classification:
-
Unlike Metals: Oxygen lacks the characteristic metallic luster, high electrical and thermal conductivity, malleability, and ductility. Metals tend to form positive ions, while oxygen predominantly forms negative ions.
-
Unlike Metalloids: Metalloids exhibit properties intermediate between metals and nonmetals, often showing variable conductivity. Oxygen's consistently poor conductivity, along with its other properties, firmly places it in the nonmetal category. Metalloids also tend to have semiconducting properties which oxygen completely lacks.
Conclusion: Oxygen – A Definitive Nonmetal
Based on its atomic structure, physical properties, and chemical behavior, oxygen is unequivocally a nonmetal. Its electron configuration, tendency to gain electrons, poor conductivity, and non-lustrous appearance all align with the characteristics of nonmetals. There is no evidence to suggest it exhibits properties consistent with either a metal or a metalloid. Its importance in biological systems and its role in chemical reactions highlight its unique and vital role as a nonmetal in our world. The debate is decisively settled: oxygen is, without a doubt, a crucial nonmetal.
Further Exploration: Oxygen's Allotropes and Reactivity
While we have established oxygen's nonmetallic nature, it's worth briefly exploring its allotropes and reactivity to gain a more complete understanding.
Allotropes of Oxygen
Oxygen exists in different allotropic forms, meaning it can exist in different molecular structures. The most common is diatomic oxygen (O₂), essential for respiration. However, another allotrope is ozone (O₃), a triatomic molecule with different properties and reactivity. Ozone is a potent oxidizing agent and plays a vital role in the stratosphere, absorbing harmful ultraviolet radiation.
Reactivity of Oxygen
Oxygen's high reactivity is a direct consequence of its nonmetallic nature and its tendency to gain electrons. It readily reacts with a wide range of elements and compounds, forming oxides. This reactivity is fundamental to many processes, including combustion, respiration, and corrosion. The oxidizing power of oxygen fuels numerous reactions and drives important chemical transformations in various environments.
Oxygen in Biological Systems
Finally, the significance of oxygen in biological systems cannot be overstated. Oxygen's role in aerobic respiration, a process that generates energy in most living organisms, is critical for survival. This fundamental biological process reinforces the importance of oxygen's properties and its unique place in the world of chemistry and biology.
In summary, the question of whether oxygen is a metal, nonmetal, or metalloid has a clear and definitive answer: oxygen is a nonmetal, a crucial element with unique properties that shape our world and the very processes of life itself.
Latest Posts
Latest Posts
-
An Introduction To General Organic And Biological Chemistry
Mar 26, 2025
-
The Cutaneous Membrane Is Also Known As
Mar 26, 2025
-
Solve The Following System Of Equations Algebraically
Mar 26, 2025
-
Why Are Tertiary Carbocations More Stable
Mar 26, 2025
-
How To Draw An Integral Sign
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Oxygen A Metal Or Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
