Is Rust A Chemical Or Physical Change
Muz Play
Mar 20, 2025 · 6 min read
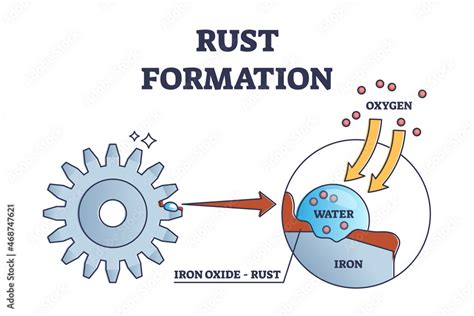
Table of Contents
Is Rust a Chemical or Physical Change? Understanding Oxidation and Corrosion
Rust, that unsightly orange-brown coating that plagues iron and steel, is a common sight. But is its formation a chemical change or a physical one? The answer, unequivocally, is chemical. Understanding why requires delving into the fascinating world of oxidation and corrosion. This comprehensive guide will explore the process of rust formation, differentiating it from physical changes and highlighting the key chemical reactions involved. We'll also explore prevention strategies and the broader implications of rust in various industries.
What is Rust?
Rust, scientifically known as iron oxide, is the result of a chemical reaction between iron and oxygen in the presence of water or moisture. It's a form of corrosion, a destructive process that affects metals and other materials. Unlike a physical change, which alters only the form or appearance of a substance without changing its chemical composition, rust fundamentally alters the chemical makeup of iron.
Key Differences Between Chemical and Physical Changes
Before diving deeper into the specifics of rust, let's solidify the fundamental difference between chemical and physical changes:
-
Physical Change: A physical change alters the form or appearance of a substance but does not change its chemical composition. Examples include melting ice, boiling water, cutting paper, or dissolving sugar in water. The substance remains the same; only its physical state or shape is altered.
-
Chemical Change: A chemical change, also known as a chemical reaction, results in the formation of one or more new substances with different chemical properties than the original substance(s). These changes are often irreversible and involve the breaking and forming of chemical bonds. Examples include burning wood, cooking an egg, or baking a cake.
The Chemistry of Rust Formation: A Detailed Look
Rust formation is a complex electrochemical process, involving several key steps and conditions:
1. Oxidation: The Core Reaction
At the heart of rust formation lies oxidation, a chemical reaction where electrons are lost by an atom or molecule. In the case of rust, iron (Fe) atoms lose electrons to oxygen (O2) molecules. This reaction is facilitated by the presence of water (H2O) or moisture, which acts as an electrolyte, enabling the flow of electrons.
The overall simplified equation for the rusting process is:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
This equation shows that iron (Fe) reacts with oxygen (O₂) and water (H₂O) to produce hydrated iron(III) oxide, commonly known as rust. However, this is a simplified representation. The actual process is far more intricate, involving multiple intermediate reactions and the formation of various iron oxides and hydroxides.
2. The Role of Electrolytes and Moisture
Water, especially when containing dissolved salts or acids, acts as an electrolyte. Electrolytes are substances that conduct electricity when dissolved in water. This conductivity is crucial for the electrochemical process of rust formation. The presence of electrolytes accelerates the rate of electron transfer between iron and oxygen.
The more acidic the water, the faster the rusting process. This explains why iron rusts faster in salty seawater compared to fresh water. The salts in seawater act as electrolytes, enhancing the conductivity and speeding up the oxidation process.
3. Anodic and Cathodic Reactions: The Electrochemical Cell
The rusting process can be visualized as an electrochemical cell, with two distinct reactions occurring at different locations:
- Anode (Oxidation): At the anode, iron loses electrons and forms iron(II) ions (Fe²⁺):
Fe(s) → Fe²⁺(aq) + 2e⁻
- Cathode (Reduction): At the cathode, oxygen gains electrons and reacts with water to form hydroxide ions (OH⁻):
O₂(g) + 2H₂O(l) + 4e⁻ → 4OH⁻(aq)
The electrons released at the anode flow through the iron to the cathode, completing the circuit. The iron(II) ions (Fe²⁺) further react with hydroxide ions (OH⁻) and oxygen to form hydrated iron(III) oxide, which is rust:
4Fe²⁺(aq) + O₂(g) + 4H₂O(l) + 8OH⁻(aq) → 4Fe(OH)₃(s)
Why Rust is a Chemical Change: Irreversibility and New Substance Formation
The key indicators that rust formation is a chemical change are:
-
Irreversibility: Once iron has rusted, it's nearly impossible to reverse the process and recover the original iron metal. You can't simply "un-rust" something by changing its physical state.
-
Formation of a New Substance: The process produces iron oxide (rust), a completely different substance with different chemical properties than iron. Rust is brittle, flaky, and has a distinctly different color and chemical composition from the original iron. This formation of a new substance is the hallmark of a chemical change.
-
Energy Transfer: The reaction between iron, oxygen, and water is exothermic, meaning it releases heat. This energy transfer is another hallmark of a chemical change.
Preventing Rust: Strategies and Techniques
Given the detrimental effects of rust, preventing its formation is crucial in various industries, from construction and automotive to marine and aerospace. Several methods are employed:
-
Protective Coatings: Applying paints, varnishes, or other coatings creates a barrier between iron and the environment, preventing contact with oxygen and water.
-
Galvanization: Coating iron with a layer of zinc protects it from rust. Zinc is more reactive than iron, so it oxidizes preferentially, sacrificing itself to protect the underlying iron.
-
Alloying: Creating alloys of iron, such as stainless steel, by adding elements like chromium and nickel, increases resistance to corrosion. These elements form a protective oxide layer that prevents further oxidation.
-
Cathodic Protection: This method uses a more reactive metal, such as magnesium or zinc, as a sacrificial anode to protect the iron structure. The more reactive metal corrodes instead of the iron.
-
Controlling the Environment: Reducing humidity and preventing exposure to acidic environments can significantly reduce the rate of rust formation.
Rust's Impact Across Industries
Rust's destructive potential has significant economic and safety implications across various sectors:
-
Construction: Rust weakens steel structures, compromising the integrity of buildings, bridges, and other infrastructure, potentially leading to catastrophic failures.
-
Automotive: Rust significantly reduces the lifespan of vehicles, causing damage to the body, chassis, and mechanical parts.
-
Marine and Offshore: Rust is a major problem for ships, offshore platforms, and underwater structures, leading to costly repairs and potential safety hazards.
-
Aerospace: Rust can compromise the structural integrity of aircraft and spacecraft components, posing serious safety risks.
Understanding the chemical nature of rust is essential for developing effective prevention and mitigation strategies. The ongoing research into corrosion science continually improves our ability to protect metal structures and extend their lifespan, mitigating the economic and safety risks associated with rust.
Conclusion: Rust – A Chemical Phenomenon with Real-World Consequences
In conclusion, rust is undeniably a chemical change. The formation of iron oxide involves a complex electrochemical process, producing a new substance with different properties than the original iron. This irreversible change is characterized by oxidation, electron transfer, and the release of energy. Understanding this chemical process is key to developing effective rust prevention strategies, crucial for maintaining the integrity of structures and equipment across various industries. The economic and safety implications of rust highlight the importance of ongoing research and the development of advanced corrosion-resistant materials and techniques.
Latest Posts
Latest Posts
-
Match Each Graph With The Corresponding Function Type
Mar 21, 2025
-
Who Was The Father Of Humanism
Mar 21, 2025
-
How To Simplify Radicals In A Fraction
Mar 21, 2025
-
A Primary Reinforcer For A Person Would Be
Mar 21, 2025
-
Cells Are The Basic Unit Of
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is Rust A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
