Is Salt And Water A Homogeneous Mixture
Muz Play
Mar 27, 2025 · 6 min read
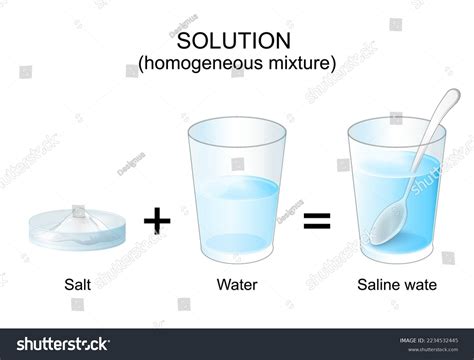
Table of Contents
Is Salt and Water a Homogeneous Mixture? A Deep Dive into Solutions
The question of whether salt and water form a homogeneous mixture is a fundamental concept in chemistry. Understanding this requires delving into the definitions of mixtures, solutions, and the properties that distinguish homogeneous from heterogeneous blends. This article will explore the science behind salt water, explaining why it's classified as a homogeneous mixture and discussing its unique properties. We’ll also touch upon the implications of this classification in various scientific fields and everyday life.
Understanding Mixtures: Homogeneous vs. Heterogeneous
Before we dive into the specifics of salt and water, let's define the key terms. A mixture is a substance composed of two or more components that are not chemically bonded. Crucially, these components retain their individual chemical properties. Mixtures can be further categorized into two main types:
Homogeneous Mixtures: Uniformity at the Microscopic Level
A homogeneous mixture is one where the components are uniformly distributed throughout the mixture. This means that at a microscopic level, the composition is consistent. No matter where you sample the mixture, you'll find the same proportion of each component. Examples include saltwater, air (a mixture of gases), and many alloys. The key is the uniformity of composition.
Heterogeneous Mixtures: Visible Differences in Composition
In contrast, a heterogeneous mixture has a non-uniform composition. You can visually distinguish different components within the mixture. Think of a salad, sand and water, or oil and vinegar. In these cases, distinct phases or regions of varying composition are clearly visible.
The Science of Saltwater: A Perfect Example of a Homogeneous Mixture
Now, let's focus on the mixture of salt (sodium chloride, NaCl) and water (H₂O). When you dissolve salt in water, the sodium chloride crystals break down into their constituent ions – sodium ions (Na⁺) and chloride ions (Cl⁻). These ions become evenly dispersed throughout the water molecules. This process is called dissolution.
The crucial point is that this dispersion is at a microscopic level. You can’t see individual sodium and chloride ions with the naked eye. A solution of salt water appears clear and transparent, with no visible distinction between the salt and water components. This uniformity is the hallmark of a homogeneous mixture.
Microscopic Uniformity: The Key to Homogeneity
The uniformity extends beyond just visual appearance. If you were to take samples from different parts of the saltwater solution, and perform a chemical analysis (such as titration), you would find that the concentration of salt ions (Na⁺ and Cl⁻) is essentially the same in each sample. This consistency at a microscopic level confirms its homogenous nature.
The Role of Water Molecules: Solvation and Dispersion
Water's ability to dissolve salt hinges on its polarity. Water molecules (H₂O) are polar, meaning they have a slightly positive end (hydrogen atoms) and a slightly negative end (oxygen atom). These polar molecules interact with the charged ions of the salt through a process called solvation. The slightly negative oxygen atoms attract the positively charged sodium ions (Na⁺), while the slightly positive hydrogen atoms attract the negatively charged chloride ions (Cl⁻). This interaction weakens the ionic bonds holding the salt crystal together, causing it to dissolve. The resulting ions are then surrounded and stabilized by water molecules, preventing them from re-forming a crystal.
Factors Affecting Saltwater Homogeneity
While saltwater is generally a homogeneous mixture, certain factors can influence its homogeneity.
Concentration of Salt: Saturation Point
The amount of salt you can dissolve in water is limited. There's a saturation point where no more salt can dissolve at a given temperature. Beyond this point, any additional salt will simply settle at the bottom, creating a heterogeneous mixture.
Temperature: Influence on Solubility
Temperature significantly affects the solubility of salt in water. Higher temperatures generally increase the solubility, meaning you can dissolve more salt before reaching saturation. This means that at high temperatures, even with a large amount of salt, you can still maintain homogeneity. Conversely, at lower temperatures, saturation occurs more quickly.
Presence of Impurities: Affecting Uniformity
The presence of impurities in the water or salt can also slightly affect homogeneity. If significant amounts of insoluble materials are present, they might create a heterogeneous mixture by settling or suspending themselves within the solution. However, in normal circumstances with reasonably pure water and salt, these effects are typically minimal.
Applications and Implications of Homogeneity
The homogeneity of saltwater is critical in many applications:
Biology and Medicine: Osmosis and Cell Function
Saltwater's homogeneity plays a vital role in biological processes, especially concerning osmosis. Cells regulate their internal environment based on the concentration of dissolved solutes, like salt, in the surrounding fluid. The uniform distribution of salt in saltwater ensures a predictable and controllable osmotic environment for cells.
Chemistry: Solutions as Reaction Media
Homogeneous solutions like saltwater are ideal media for chemical reactions because the uniform distribution of reactants ensures consistent reaction rates and product yields. In contrast, heterogeneous reactions can have unpredictable outcomes due to uneven distribution.
Oceanography: Understanding Ocean Currents and Salinity
The properties of saltwater, its homogeneity and its salinity are fundamental to understanding ocean currents, marine ecosystems, and the global water cycle. Salinity gradients influence ocean circulation patterns and the distribution of marine life.
Food Science: Preservation and Flavor
Saltwater is used extensively in food preservation (pickling, brining), where its homogeneity ensures uniform salt concentration throughout the food, inhibiting microbial growth. It also contributes to flavor profiles in many culinary applications.
Beyond Saltwater: Other Examples of Homogeneous Mixtures
The principles we've discussed regarding saltwater apply to many other homogeneous mixtures:
- Sugar water: Sugar dissolves in water in a similar manner to salt, forming a homogeneous solution.
- Air: A mixture of gases (oxygen, nitrogen, carbon dioxide, etc.), with the components evenly distributed.
- Brass: An alloy of copper and zinc, where the atoms are uniformly dispersed.
- Steel: An alloy of iron and carbon, exhibiting homogeneous properties throughout the material.
Conclusion: The Importance of Homogeneity in Science and Everyday Life
The classification of salt and water as a homogeneous mixture is more than just a label; it reflects a fundamental understanding of the interplay between molecules and ions in solution. This understanding has far-reaching consequences in various scientific disciplines and daily applications. The uniform distribution of components in a homogeneous mixture leads to predictable and controllable properties, making these mixtures essential in countless processes and systems. Understanding the characteristics of homogeneous mixtures like saltwater allows us to better manipulate and utilize these mixtures in numerous ways. From the intricate workings of cellular processes to the vast dynamics of ocean currents, the concept of homogeneity plays a crucial role in our understanding of the natural world and our ability to harness its properties for practical purposes.
Latest Posts
Latest Posts
-
Difference Between The Autonomic And Somatic Nervous System
Mar 30, 2025
-
Examples Of Literary Analysis Thesis Statements
Mar 30, 2025
-
Why Might Two Elements Possess Similar Chemical Properties
Mar 30, 2025
-
Where Does Water Enter The Plant
Mar 30, 2025
-
Cytochrome C Carries How Many Electrons
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Salt And Water A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
