Cytochrome C Carries How Many Electrons
Muz Play
Mar 30, 2025 · 6 min read
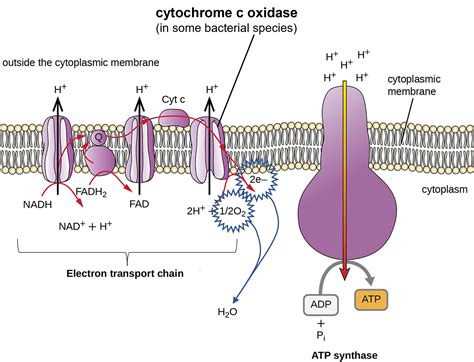
Table of Contents
Cytochrome c: A Deep Dive into its Electron-Carrying Capacity
Cytochrome c, a crucial component of the electron transport chain (ETC), plays a vital role in cellular respiration. Understanding its function hinges on comprehending its electron-carrying capacity. This article delves into the intricacies of cytochrome c, exploring its structure, function, and the precise number of electrons it can transport. We will also discuss the implications of its electron transfer capabilities within the context of cellular respiration and its overall importance in biological systems.
The Structure of Cytochrome c: A Foundation for Function
Cytochrome c is a small, heme-containing protein found in the intermembrane space of mitochondria in eukaryotes and the periplasmic space of bacteria. Its structure is remarkably conserved across species, reflecting its essential role in cellular processes. The protein's structure is crucial to its ability to accept and donate electrons.
Heme Group: The Electron Shuttle
At the heart of cytochrome c's function lies its heme group, a porphyrin ring coordinated to an iron ion (Fe). This iron ion is the key player in electron transport. The iron can exist in two oxidation states: Fe<sup>2+</sup> (ferrous) and Fe<sup>3+</sup> (ferric). The ability of the iron ion to switch between these two oxidation states is the foundation of cytochrome c's electron-carrying capacity. The heme group is tightly bound within a hydrophobic pocket of the protein, shielding it from the aqueous environment and optimizing its redox properties.
Protein Scaffold: Maintaining the Heme Environment
The protein scaffold surrounding the heme group is not merely structural support. It plays a critical role in controlling the heme's redox potential and ensuring efficient electron transfer. The amino acid residues surrounding the heme group fine-tune the environment, influencing the ease with which the iron ion accepts or donates an electron. Specific amino acid interactions stabilize the different oxidation states of the iron, preventing unwanted side reactions and ensuring a controlled electron transfer process. This precisely controlled environment is essential for the efficient functioning of the ETC.
Cytochrome c and Electron Transfer: A One-Electron Affair
Cytochrome c carries only one electron at a time. This single-electron transfer is a fundamental aspect of its function within the electron transport chain. The process involves the reduction of the ferric iron (Fe<sup>3+</sup>) to ferrous iron (Fe<sup>2+</sup>) when it accepts an electron, and the oxidation of the ferrous iron back to ferric iron when it donates an electron. This cyclical process of reduction and oxidation is central to its role in facilitating the flow of electrons through the respiratory chain.
The specificity of this one-electron transfer is carefully orchestrated by the protein's structure. The protein's architecture precisely positions the heme group and nearby amino acid residues to facilitate the binding and release of a single electron without allowing the acceptance or donation of more. This precise control is critical for avoiding the formation of potentially damaging reactive oxygen species (ROS), which can arise from incomplete electron transfers.
Cytochrome c's Role in the Electron Transport Chain: A Critical Link
Cytochrome c acts as a mobile electron carrier within the mitochondrial intermembrane space (or periplasmic space in bacteria). It receives an electron from cytochrome bc1 complex (Complex III) and then transfers it to cytochrome c oxidase (Complex IV). This transfer of electrons from one complex to another is a crucial step in oxidative phosphorylation.
The Electron Flow: From Complex III to Complex IV
The electron transfer from cytochrome bc1 complex to cytochrome c is highly regulated. The precise orientation and interactions between these two complexes are essential for efficient electron transfer. This controlled process ensures that electrons are passed along the chain in a unidirectional manner, preventing backflow and maximizing the efficiency of ATP synthesis.
The interaction of cytochrome c with cytochrome c oxidase is similarly controlled. Specific binding sites and conformational changes ensure the correct transfer of electrons to the next component in the respiratory chain. This intricate dance of molecular interactions is essential for the proper functioning of the ETC.
Implications of Cytochrome c's One-Electron Transfer: Efficiency and Regulation
The one-electron transfer mechanism employed by cytochrome c offers several critical advantages:
- Controlled Electron Flow: This precise control prevents the generation of harmful ROS.
- High Efficiency: The sequential transfer of single electrons maximizes the efficiency of energy conversion.
- Regulation: The one-electron transfer process allows for precise regulation of electron flow in response to cellular needs.
Beyond the Basics: Cytochrome c and Apoptosis
While its role in the ETC is paramount, cytochrome c's functions extend beyond cellular respiration. In the context of apoptosis (programmed cell death), cytochrome c's release from the mitochondria acts as a crucial signaling molecule. This release initiates a cascade of events leading to cell demise.
Cytochrome c's Dual Role: Life and Death
The dual role of cytochrome c in both energy production and programmed cell death highlights its critical position in maintaining cellular homeostasis. The regulation of cytochrome c release from the mitochondria is tightly controlled, preventing premature apoptosis and ensuring proper cellular function. Disruptions in this regulatory mechanism can lead to various pathological conditions.
Studying Cytochrome c: Techniques and Discoveries
Research on cytochrome c has utilized a variety of techniques to understand its structure and function:
- X-ray crystallography: This technique has provided high-resolution images of cytochrome c's structure, revealing the details of its heme environment and protein scaffold.
- Spectroscopy: Various spectroscopic methods, such as UV-Vis and EPR spectroscopy, have been used to study the redox properties of cytochrome c and its interactions with other molecules.
- Electrochemistry: Electrochemical methods provide quantitative information about the redox potential and electron transfer kinetics of cytochrome c.
- Molecular dynamics simulations: Computational simulations provide insights into the dynamic aspects of cytochrome c's function and interactions.
These techniques have been crucial in elucidating the precise mechanisms of cytochrome c's electron transfer and its interaction with other proteins in the electron transport chain and apoptotic pathways.
Conclusion: Cytochrome c – A Masterpiece of Molecular Engineering
Cytochrome c, with its precisely engineered structure and its unique one-electron carrying capacity, represents a remarkable example of molecular evolution. Its crucial role in both cellular respiration and programmed cell death highlights its central importance to cellular life. Further research continues to unravel the complexities of this vital protein, providing a deeper understanding of its function and the broader implications for human health and disease. The precise control of one-electron transfer by cytochrome c exemplifies the sophistication of biological systems and their ability to harness this process for fundamental life functions. Understanding this single-electron transfer mechanism is key to understanding the intricate workings of cellular respiration and its importance in sustaining life.
Latest Posts
Latest Posts
-
The Urinary System Regulates Blood Volume And Pressure By
Apr 01, 2025
-
Genomics Can Be Used In Agriculture To
Apr 01, 2025
-
What Is Used For Measuring Mass
Apr 01, 2025
-
Second Moment Of Inertia Parallel Axis Theorem
Apr 01, 2025
-
Single Displacement Reaction Examples In Real Life
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Cytochrome C Carries How Many Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
