Single Displacement Reaction Examples In Real Life
Muz Play
Apr 01, 2025 · 6 min read
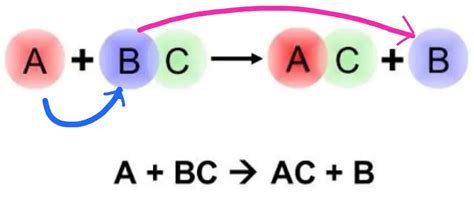
Table of Contents
Single Displacement Reactions: Everyday Examples You Encounter
Single displacement reactions, also known as single replacement reactions, are a fundamental type of chemical reaction where one element replaces another element in a compound. This process involves a more reactive element displacing a less reactive element from its compound, resulting in the formation of a new compound and a free element. Understanding these reactions is crucial in various fields, from industrial processes to everyday occurrences. This article will delve into the intricacies of single displacement reactions, explaining the underlying principles and providing numerous real-life examples.
Understanding the Basics of Single Displacement Reactions
The general form of a single displacement reaction is represented as:
A + BC → AC + B
Where:
- A is a more reactive element.
- B is a less reactive element.
- BC is a compound.
- AC is a new compound formed.
For a single displacement reaction to occur, element A must be more reactive than element B according to the reactivity series. The reactivity series is a list of elements arranged in order of their decreasing reactivity. A more reactive element will readily displace a less reactive element from its compound. This reactivity is determined by factors like electronegativity, ionization energy, and standard reduction potential.
Essential Conditions for a Single Displacement Reaction
Several conditions are necessary for a successful single displacement reaction:
-
Reactivity Difference: A significant difference in reactivity between element A and element B is crucial. A highly reactive element will readily replace a less reactive one.
-
Appropriate Medium: Some reactions require specific solvents or conditions, like an aqueous solution or heating, to facilitate the reaction.
-
Contact Between Reactants: The reactants must come into contact for the reaction to proceed. This often involves mixing the reactants in a suitable medium.
Real-Life Applications and Examples of Single Displacement Reactions
Single displacement reactions are surprisingly common in everyday life, often unnoticed. Let's explore several examples across various fields:
1. Rusting of Iron (Corrosion):
Reaction: 4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
This is a classic example, although not strictly a simple single displacement. Oxygen in the air displaces some of the electrons from iron atoms, leading to the formation of iron(III) oxide-hydroxide, commonly known as rust. This process weakens the iron structure and is a major concern in construction and engineering. Preventing rust is a significant technological challenge, leading to the development of various protective coatings and alloys. The presence of water accelerates the process.
2. Extraction of Metals:
The extraction of many metals from their ores often involves single displacement reactions. A more reactive metal is used to displace a less reactive metal from its compound.
Example: The extraction of copper from its ore using iron:
Reaction: Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
Here, iron, being more reactive than copper, displaces copper from copper(II) sulfate solution, resulting in the formation of iron(II) sulfate and solid copper. This is a common method for extracting copper from its compounds.
Similarly, the extraction of other metals involves similar reactions. The choice of reducing agent (the more reactive metal) depends on the reactivity of the metal being extracted.
3. The Production of Hydrogen Gas:
Hydrogen gas is often produced through single displacement reactions involving the reaction of metals with acids or water.
Example: The reaction of zinc with hydrochloric acid:
Reaction: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
In this reaction, zinc, a more reactive metal, displaces hydrogen from hydrochloric acid, producing zinc chloride and hydrogen gas. This reaction is commonly used in laboratories to generate hydrogen gas for various experiments.
4. Reactions in Photography:
Certain photographic processes involve single displacement reactions. For example, the development of photographic film involves the reduction of silver halide crystals to metallic silver using a reducing agent.
Simplified Reaction: AgBr(s) + Reducing Agent → Ag(s) + Br⁻(aq) + Oxidized Reducing Agent
The reducing agent displaces the silver from the silver halide, resulting in the formation of metallic silver, which constitutes the photographic image. The exact reducing agent and reaction details vary depending on the specific film and developer used.
5. The Use of Silver Polish:
Silver tarnish is primarily silver sulfide (Ag₂S), a black coating formed due to the reaction of silver with sulfur compounds in the environment. Many silver polishes employ single displacement reactions to remove this tarnish.
Simplified Reaction: Ag₂S(s) + Reducing Agent → 2Ag(s) + S(s) + Oxidized Reducing Agent
The reducing agent in the polish reacts with the silver sulfide, reducing it back to shiny metallic silver. The sulfur is typically removed in the process.
6. Electroplating:
Electroplating is a widely used industrial process where a thin layer of a metal is deposited on another metal surface. This often involves a single displacement reaction at the cathode.
Example: Electroplating copper onto iron:
At the cathode (negative electrode): Cu²⁺(aq) + 2e⁻ → Cu(s)
Copper ions from the solution are reduced and deposited onto the iron surface, plating it with a layer of copper. This process protects the iron from corrosion and enhances its appearance.
7. Water Purification:
Some water purification processes utilize single displacement reactions to remove unwanted substances, like heavy metals.
Example: Using a more reactive metal to precipitate heavy metal ions:
For instance, using iron filings to remove lead from water: Pb²⁺(aq) + Fe(s) → Pb(s) + Fe²⁺(aq)
(Note: The actual process might be more complex and involve multiple steps, but the core principle involves displacement).
8. Reactions in Batteries:
Many types of batteries utilize single displacement reactions to generate electricity. The flow of electrons during the redox reaction powers the device.
Example: The reaction in a simple zinc-copper battery:
Zn(s) → Zn²⁺(aq) + 2e⁻ (Oxidation at the anode)
Cu²⁺(aq) + 2e⁻ → Cu(s) (Reduction at the cathode)
Zinc loses electrons (oxidation), while copper ions gain electrons (reduction). The flow of electrons generates an electric current.
9. Thermite Reaction:
While a more complex reaction, the thermite reaction involves the displacement of a metal from its oxide by another metal, typically aluminum.
Reaction: Fe₂O₃(s) + 2Al(s) → 2Fe(l) + Al₂O₃(s)
Aluminum displaces iron from iron(III) oxide, producing molten iron and aluminum oxide. This highly exothermic reaction is used in welding and other specialized applications.
10. Reactions in Biology:
Some biological processes also involve single displacement reactions. For example, certain enzyme-catalyzed reactions can be viewed as a type of single displacement, where a substrate molecule replaces a portion of the enzyme-substrate complex. However, these are often more complex than the simple inorganic examples discussed above.
Conclusion: The Ubiquity of Single Displacement Reactions
Single displacement reactions are not just confined to chemistry labs; they are integral to numerous aspects of our daily lives, from the corrosion of metals to the extraction of valuable resources and the operation of batteries. Understanding these fundamental reactions helps us appreciate the chemical processes shaping our world and provides insights for developing new technologies and solving practical problems. By recognizing the underlying principles and the various real-life manifestations of these reactions, we gain a deeper understanding of the chemical dynamics influencing our daily experiences.
Latest Posts
Latest Posts
-
How Do You Square A Radical
Apr 02, 2025
-
Was Adolf Hitler A Good Leader
Apr 02, 2025
-
Is Cholesterol A Carbohydrate Protein Or Lipid
Apr 02, 2025
-
Pick A Number Between 1 And 51
Apr 02, 2025
-
Do Protists Have Membrane Bound Organelles
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Single Displacement Reaction Examples In Real Life . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
