Is Salt Water A Mixture Or Pure Substance
Muz Play
Mar 21, 2025 · 5 min read
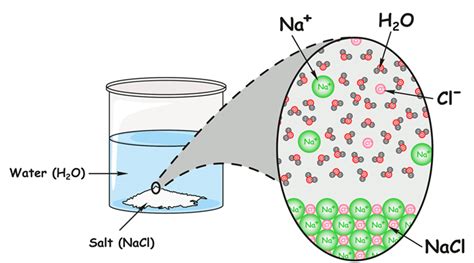
Table of Contents
Is Salt Water a Mixture or a Pure Substance? A Deep Dive into Solutions
The question, "Is saltwater a mixture or a pure substance?" seems simple enough, but it opens a door to a fascinating exploration of chemistry and the properties of matter. The answer, in short, is that saltwater is a mixture. However, understanding why requires delving into the fundamental differences between mixtures and pure substances, and specifically the characteristics of solutions. This article will provide a comprehensive analysis, exploring the topic from various perspectives, including the macroscopic and microscopic levels, and touching upon relevant concepts such as homogeneous and heterogeneous mixtures, solutions, solutes, and solvents. We'll also address some common misconceptions and explore the implications of this classification.
Understanding Pure Substances and Mixtures
Before we dive into the specifics of saltwater, let's establish a clear understanding of the core concepts: pure substances and mixtures.
Pure Substances: The Building Blocks
A pure substance is a form of matter that has a fixed chemical composition and distinct properties. It cannot be separated into other substances by physical methods (like filtration or distillation). Pure substances can be either elements or compounds.
-
Elements: These are the fundamental building blocks of matter, appearing on the periodic table. They cannot be broken down into simpler substances by chemical means. Examples include oxygen (O₂), hydrogen (H₂), and gold (Au).
-
Compounds: These are formed when two or more elements chemically combine in a fixed ratio. They can only be separated into their constituent elements through chemical reactions. Examples include water (H₂O), sodium chloride (NaCl - table salt), and carbon dioxide (CO₂).
Mixtures: A Blend of Substances
A mixture, on the other hand, is a combination of two or more substances that are not chemically bonded. The substances retain their individual properties and can be separated by physical methods. Mixtures can be either homogeneous or heterogeneous.
-
Homogeneous Mixtures: In these mixtures, the components are uniformly distributed throughout the mixture. This means the composition is the same throughout, and you can't visually distinguish the individual components. Saltwater is a classic example of a homogeneous mixture.
-
Heterogeneous Mixtures: In these mixtures, the components are not uniformly distributed. You can visually distinguish the different parts of the mixture. Examples include sand and water, or a salad.
Saltwater: A Detailed Analysis
Now let's focus on saltwater. Saltwater is a solution formed when table salt (sodium chloride, NaCl) dissolves in water (H₂O). Understanding this requires understanding the concept of solutions.
Solutions: A Special Type of Mixture
A solution is a type of homogeneous mixture where one substance (the solute) dissolves in another substance (the solvent). The solute is typically present in a smaller amount than the solvent. In saltwater:
- Solute: Sodium chloride (NaCl) - the salt.
- Solvent: Water (H₂O) - the water.
The dissolving process involves the water molecules surrounding and separating the sodium and chloride ions from each other. This process is called solvation or hydration in the case of water. The resulting solution is clear and transparent because the salt ions are evenly dispersed at the molecular level, hence its homogeneous nature.
Why Saltwater Isn't a Pure Substance
Saltwater is clearly a mixture because:
-
Variable Composition: The ratio of salt to water can vary. You can have a highly concentrated saltwater solution (lots of salt) or a dilute solution (little salt). A pure substance has a fixed composition.
-
Separation by Physical Methods: The salt and water in saltwater can be separated using physical methods such as evaporation. Simply heating the saltwater will cause the water to evaporate, leaving behind the salt crystals. This is a clear indication it's not a compound.
-
Retention of Individual Properties: Even though the salt dissolves, its properties are not lost. If you evaporate the water, you recover the salt, and it retains its characteristic taste and crystalline structure. The water also retains its properties. This distinguishes it from a chemical reaction where new substances with different properties are formed.
Common Misconceptions
Several misconceptions surround the classification of saltwater:
-
"It looks pure": The clarity and uniformity of saltwater can be misleading. Remember, homogeneous mixtures, like solutions, appear uniform at the macroscopic level but are composed of different substances at the microscopic level.
-
"The salt disappears": The salt doesn't disappear; it simply dissolves and disperses evenly among the water molecules. The ions are still present, albeit individually dispersed and not forming a new chemical bond.
-
"It's a compound because it's formed from two substances": The key difference lies in the nature of the interaction between the substances. In a mixture, the substances are physically combined, not chemically bonded. In a compound, a chemical reaction leads to the formation of a new substance with distinct properties different from its components.
Beyond Saltwater: Exploring Other Solutions
Understanding saltwater as a mixture helps us understand many other solutions prevalent in our daily lives. These include:
- Sugar water: Sugar (sucrose) dissolved in water.
- Air: A mixture of various gases (oxygen, nitrogen, carbon dioxide, etc.). While not a solution in the strictest sense (since gases don't "dissolve" in the same way as solids in liquids), it's a homogeneous mixture with variable composition.
- Seawater: Besides salt, seawater contains numerous other dissolved minerals and substances, making it an even more complex mixture.
- Many alloys: Some alloys, like brass (copper and zinc), are homogeneous mixtures.
Conclusion: Saltwater – A Homogeneous Mixture
To reiterate, saltwater is unequivocally a homogeneous mixture, specifically a solution. Its variable composition, the ability to separate its components through physical means, and the retention of individual properties by its constituents clearly distinguish it from a pure substance. Understanding this distinction is fundamental to grasping the basics of chemistry and the diverse ways matter exists in our world. The implications of classifying saltwater correctly extends far beyond simple terminology; it's crucial for numerous scientific disciplines, including oceanography, chemistry, and environmental science. This seemingly simple question opens a pathway to a richer appreciation of the complex interactions and properties of matter.
Latest Posts
Latest Posts
-
How Many Neutrons Does K Have
Mar 27, 2025
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
-
Song Lyrics Ode To Billy Joe
Mar 27, 2025
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Is Salt Water A Mixture Or Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
