Is Salt Water A Substance Or Mixture
Muz Play
Mar 19, 2025 · 6 min read
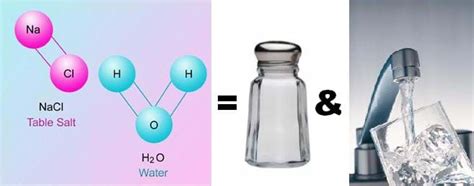
Table of Contents
Is Salt Water a Substance or a Mixture? A Deep Dive into Chemical Classification
The question of whether saltwater is a substance or a mixture often arises in chemistry discussions. While seemingly simple, the answer delves into the fundamental principles of chemical classification and the properties of matter. This comprehensive guide will explore the characteristics of substances and mixtures, examining saltwater through this lens to definitively answer this question and delve into related concepts.
Understanding Substances and Mixtures
Before classifying saltwater, let's establish a clear understanding of the terms "substance" and "mixture." These are fundamental categories in chemistry used to classify matter based on its composition and properties.
Substances: The Pure Forms of Matter
A substance, also known as a pure substance, is a form of matter that has a fixed chemical composition and uniform properties throughout. This means that regardless of the sample size or source, a substance will always possess the same chemical makeup and physical properties. Substances can be further categorized into two types:
-
Elements: These are the simplest forms of matter, composed of only one type of atom. Examples include oxygen (O), hydrogen (H), and iron (Fe). Elements cannot be broken down into simpler substances through chemical means.
-
Compounds: These are substances formed when two or more elements chemically combine in a fixed ratio. This combination results in a new substance with properties distinct from its constituent elements. Examples include water (H₂O), sodium chloride (NaCl – table salt), and carbon dioxide (CO₂). Compounds can be broken down into their constituent elements through chemical reactions.
Mixtures: A Blend of Substances
A mixture is a combination of two or more substances that are physically combined but not chemically bonded. Crucially, the components of a mixture retain their individual properties and can be separated using physical methods, such as filtration, distillation, or evaporation. Mixtures can be further categorized into:
-
Homogeneous Mixtures: These mixtures have a uniform composition throughout. This means that the different components are evenly distributed, and the mixture appears the same throughout. Examples include saltwater, air, and sugar dissolved in water.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition. The different components are not evenly distributed, and different parts of the mixture have different properties. Examples include sand and water, oil and water, and a salad.
Classifying Saltwater: A Mixture, Not a Substance
Now, let's apply this knowledge to saltwater. Saltwater is a solution composed primarily of water (H₂O) and sodium chloride (NaCl). The sodium chloride dissolves in the water, creating a homogenous mixture where the salt ions (Na⁺ and Cl⁻) are evenly dispersed throughout the water molecules.
Key characteristics of saltwater that indicate it's a mixture:
-
Variable Composition: The ratio of salt to water in saltwater can vary significantly depending on the source. Ocean water has a different salt concentration than water from a salt lake or a bay. This variability in composition is a hallmark of mixtures, unlike substances that maintain a fixed ratio.
-
Retention of Individual Properties: Although the salt dissolves in water, it doesn't lose its chemical identity. The salt can be recovered from the saltwater through physical methods like evaporation, leaving behind the solid sodium chloride crystals. Similarly, the water retains its properties.
-
Separation by Physical Means: The components of saltwater can be separated using simple physical techniques like evaporation or distillation. Evaporation leaves behind salt crystals, while distillation separates the water from the salt. This is a defining characteristic of mixtures.
Therefore, saltwater is definitively classified as a homogeneous mixture, not a substance. It's a combination of two substances – water and sodium chloride – that retain their individual properties and can be separated by physical means.
Deeper Dive into the Properties of Saltwater
Understanding saltwater as a mixture allows us to further explore its properties, including its:
1. Density:
Saltwater is denser than freshwater due to the presence of dissolved salts. The added mass of the salt ions increases the overall density of the solution. This difference in density is critical in various natural phenomena, like ocean currents and marine life distribution.
2. Boiling Point:
The boiling point of saltwater is higher than that of pure water. The dissolved salts interfere with the water molecules' ability to transition to the gaseous phase, requiring a higher temperature to achieve boiling.
3. Freezing Point:
Conversely, the freezing point of saltwater is lower than that of pure water. The dissolved salts disrupt the formation of the crystalline structure of ice, requiring a lower temperature for freezing. This is why saltwater doesn't freeze as easily as freshwater.
4. Conductivity:
Saltwater is a good conductor of electricity. This is because the dissolved salt ions carry an electric charge, facilitating the flow of electricity through the solution. This property is exploited in various applications, such as desalination processes.
5. Osmosis:
Saltwater plays a critical role in osmosis, the movement of water across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. This process is fundamental to biological systems and is affected by the salinity of the solution.
Beyond Sodium Chloride: The Complexity of Seawater
While the simplified model of saltwater as a mixture of water and sodium chloride is helpful, seawater is far more complex. It contains a vast array of dissolved ions, gases, and organic molecules. These include:
- Magnesium (Mg²⁺): A significant component of seawater, contributing to its overall salinity.
- Calcium (Ca²⁺): Essential for marine organisms to build shells and skeletons.
- Potassium (K⁺): An important ion involved in various biological processes.
- Sulfate (SO₄²⁻): A major anion in seawater.
- Bicarbonate (HCO₃⁻): Plays a crucial role in ocean acidification.
- Dissolved gases: Oxygen (O₂), carbon dioxide (CO₂), and nitrogen (N₂) are present in varying concentrations.
- Organic matter: A complex mixture of dissolved organic compounds from living organisms and their decomposition.
This complexity underlines that seawater is a dynamic and multifaceted mixture, not just a simple combination of water and salt. Understanding the composition of seawater is crucial for various scientific disciplines, including oceanography, marine biology, and environmental science.
Practical Applications and Implications
Understanding the nature of saltwater as a mixture has far-reaching practical applications and implications:
-
Desalination: The process of removing salt from saltwater to produce freshwater for drinking and irrigation relies on understanding the properties of saltwater mixtures. Different techniques are employed, depending on the salinity and the desired level of purity.
-
Oceanography: Oceanographers study the composition and properties of seawater to understand ocean currents, marine ecosystems, and the impact of climate change on the ocean.
-
Marine Biology: Marine organisms have adapted to specific salinity levels, and understanding the composition of seawater is essential for studying their physiology and ecology.
-
Environmental Science: The study of saltwater and its pollution helps scientists understand and mitigate the effects of human activities on marine environments.
Conclusion: Saltwater – A Homogeneous Mixture of Significance
In conclusion, saltwater is unequivocally a homogeneous mixture, not a substance. Its variable composition, the retention of individual properties by its components, and the ability to separate these components by physical means all support this classification. While a simplified model views saltwater as a mixture of water and sodium chloride, the reality is far richer, encompassing a complex array of dissolved ions, gases, and organic molecules. Understanding the properties and composition of this vital mixture is critical across diverse scientific fields and holds significant practical implications for humanity's interaction with and dependence on our oceans.
Latest Posts
Latest Posts
-
Does Fluorine Cause 13c Nmr Splitting
Mar 19, 2025
-
What Are The Three Functions Of Roots
Mar 19, 2025
-
When To Use Henderson Hasselbalch Equation
Mar 19, 2025
-
Systems Of Linear Equations Application Problems
Mar 19, 2025
-
Find The Inverse Laplace Transform Of
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Salt Water A Substance Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
