When To Use Henderson Hasselbalch Equation
Muz Play
Mar 19, 2025 · 6 min read
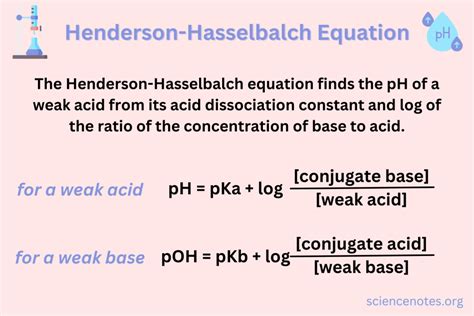
Table of Contents
When to Use the Henderson-Hasselbalch Equation: A Comprehensive Guide
The Henderson-Hasselbalch equation is a cornerstone of acid-base chemistry, providing a crucial link between pH, pKa, and the concentrations of an acid and its conjugate base. Understanding when and how to apply this equation is essential for anyone working in chemistry, biochemistry, or related fields. This comprehensive guide delves deep into the applications, limitations, and nuances of the Henderson-Hasselbalch equation.
What is the Henderson-Hasselbalch Equation?
The Henderson-Hasselbalch equation is expressed as:
pH = pKa + log([A⁻]/[HA])
Where:
- pH: The negative logarithm of the hydrogen ion concentration ([H⁺]), representing the acidity or alkalinity of a solution.
- pKa: The negative logarithm of the acid dissociation constant (Ka), representing the strength of the acid. A lower pKa indicates a stronger acid.
- [A⁻]: The concentration of the conjugate base.
- [HA]: The concentration of the weak acid.
When to Use the Henderson-Hasselbalch Equation: Key Applications
The Henderson-Hasselbalch equation finds widespread application in various scenarios, but its effective use hinges on understanding its underlying assumptions and limitations. Let's explore the key situations where this equation proves invaluable:
1. Calculating the pH of a Buffer Solution
This is arguably the most common and crucial application. A buffer solution resists changes in pH upon addition of small amounts of acid or base. It typically consists of a weak acid (HA) and its conjugate base (A⁻). The Henderson-Hasselbalch equation allows for the precise calculation of the pH of such a buffer solution, given the pKa of the weak acid and the concentrations of the acid and its conjugate base.
Example: Calculating the pH of an acetic acid/acetate buffer. Knowing the pKa of acetic acid and the concentrations of acetic acid and acetate ions, the equation directly provides the buffer's pH. This is crucial in biological systems, where maintaining a stable pH is vital.
2. Determining the Ratio of Acid to Conjugate Base for a Desired pH
The equation can be rearranged to determine the required ratio of [A⁻]/[HA] to achieve a specific pH in a buffer solution. This is particularly useful in preparing buffer solutions with a predetermined pH for experiments or applications requiring a specific pH range.
Example: A researcher needs a buffer solution at pH 5.0 using a weak acid with a pKa of 4.8. By using the rearranged equation, the researcher can calculate the necessary ratio of conjugate base to weak acid to achieve the desired pH. This ensures the buffer effectively maintains the target pH.
3. Analyzing Titration Curves
Titration curves graphically represent the change in pH as a strong acid or base is added to a weak acid or base solution. The Henderson-Hasselbalch equation helps interpret these curves, particularly in the buffer region. At the half-equivalence point of a titration (where half the weak acid has been neutralized), [A⁻] = [HA], and the equation simplifies to pH = pKa. This provides a direct method for determining the pKa of the weak acid from the titration curve.
Example: In a titration of acetic acid with a strong base, the point on the titration curve where the pH equals the pKa of acetic acid corresponds to the half-equivalence point.
4. Understanding Drug Absorption and Distribution
In pharmacology and physiology, the Henderson-Hasselbalch equation plays a critical role in understanding drug absorption and distribution within the body. Many drugs are weak acids or bases, and their degree of ionization depends on the pH of the surrounding environment. The equation helps predict the proportion of ionized and non-ionized forms of the drug, which influences its absorption, distribution, and excretion. Non-ionized forms generally cross cell membranes more readily.
Example: A weak acid drug will be largely non-ionized in acidic environments (like the stomach) and readily absorbed. In more alkaline environments (like the intestines), it becomes more ionized and absorption may be reduced.
5. Predicting the Solubility of Weak Acids and Bases
The ionization state of weak acids and bases significantly affects their solubility in different environments. The Henderson-Hasselbalch equation enables prediction of the solubility of a weak acid or base based on the pH of the solution and the pKa of the compound.
Example: A weak acid drug with a pKa close to the pH of the surrounding solution will have reduced solubility as the ionized form often has lower solubility than the neutral form.
6. Environmental Chemistry Applications
The equation finds use in analyzing the pH of natural water bodies, soil solutions, and other environmental systems. Understanding the acid-base equilibria of various substances in these systems is crucial for environmental monitoring and remediation.
Limitations of the Henderson-Hasselbalch Equation
While incredibly useful, the Henderson-Hasselbalch equation is not universally applicable. Its limitations must be carefully considered:
-
It's only accurate for weak acids and bases: The equation assumes that the acid is weak and that its dissociation is not significant enough to significantly alter the overall concentration of the acid and its conjugate base. For strong acids and bases, the equation is not applicable.
-
It assumes that the activity coefficients are close to 1: The equation utilizes concentrations instead of activities. This approximation is reasonable in dilute solutions, but in concentrated solutions, activity coefficients deviate from 1, affecting accuracy.
-
It's not applicable at extreme pH values: At very high or low pH values, the assumptions underlying the equation break down. For example, at very high pH, virtually all the acid will be deprotonated, and the equation becomes less reliable.
Advanced Considerations and Applications
Beyond the basic applications, the Henderson-Hasselbalch equation finds use in more complex scenarios:
-
Isoelectric Point Calculation: In biochemistry, the isoelectric point (pI) of a protein is the pH at which the net charge of the protein is zero. This point is crucial in various applications, including protein purification and electrophoresis. Calculations involving multiple ionizable groups in a molecule require modifications of the basic equation.
-
Polyprotic Acid Systems: While the basic equation deals with monoprotic acids (acids with one ionizable proton), extensions exist for polyprotic acids (acids with multiple ionizable protons). The calculation becomes more complex but still relies on the principles underlying the Henderson-Hasselbalch equation.
-
Enzyme Kinetics: Some enzyme reactions involve acid-base catalysis, where the pH of the environment influences the reaction rate. The Henderson-Hasselbalch equation can aid in understanding the relationship between pH and the catalytic activity of the enzyme.
Conclusion
The Henderson-Hasselbalch equation is an indispensable tool in a wide range of chemical and biological applications. Its ability to link pH, pKa, and the concentrations of acids and their conjugate bases makes it invaluable for understanding and manipulating acid-base equilibria. While limitations exist, understanding these limitations enhances the effective use of the equation. By mastering this equation and its applications, one gains significant insight into the intricate world of acid-base chemistry and its relevance to diverse fields. Remember to always carefully consider the assumptions and limitations before applying the equation to ensure accurate and meaningful results.
Latest Posts
Latest Posts
-
Inverted Vs Everted Palindromic Dna Sequence Example
Mar 19, 2025
-
What Makes Up Most Of The Mass Of An Atom
Mar 19, 2025
-
Policy Implementation Refers To The Bureaucratic Function Of
Mar 19, 2025
-
Example Of A Formal Lab Report For Chemistry
Mar 19, 2025
-
The Fluid Filled Area Within The Chloroplast Is Called The
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about When To Use Henderson Hasselbalch Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
