Is Silver A Metal Metalloid Or Nonmetal
Muz Play
Mar 24, 2025 · 5 min read
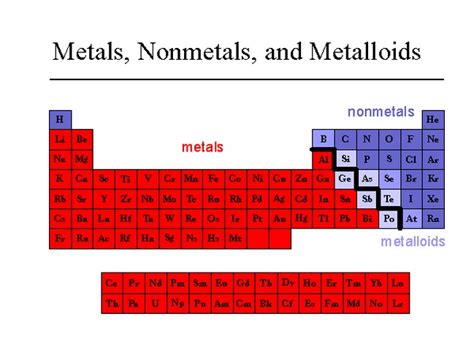
Table of Contents
Is Silver a Metal, Metalloid, or Nonmetal? A Comprehensive Exploration
Silver, a lustrous white metal known for its conductivity and malleability, has held a significant place in human history, from ancient coinage to modern electronics. But where does it sit on the periodic table's elemental classification? Is silver a metal, metalloid, or nonmetal? The answer, unequivocally, is metal. This article will delve deep into the properties and characteristics of silver to definitively establish its classification and explore its unique position within the metallic family.
Understanding the Classification of Elements
Before diving into the specifics of silver, let's briefly review the three main categories of elements: metals, metalloids, and nonmetals. These categories are based on elements' physical and chemical properties, primarily their electronegativity, ionization energy, and conductivity.
Metals
Metals are characterized by their:
- High electrical conductivity: They readily conduct electricity due to the ease with which electrons can move through their structure.
- High thermal conductivity: They efficiently transfer heat.
- Malleability and ductility: They can be easily shaped (hammered into sheets) and drawn into wires.
- Luster: They possess a shiny appearance.
- High density: They are generally dense compared to nonmetals.
- Low ionization energy: They readily lose electrons to form positive ions.
Metalloids (Semimetals)
Metalloids exhibit properties that lie between those of metals and nonmetals. They often:
- Have intermediate electrical conductivity: Their conductivity is variable and can be influenced by factors like temperature and pressure. They are often semiconductors.
- Exhibit both metallic and nonmetallic properties: Their behavior can vary depending on the specific conditions.
- Are brittle and less malleable than metals: They are not easily shaped.
Nonmetals
Nonmetals are typically:
- Poor conductors of electricity and heat: They resist the flow of both.
- Brittle: They lack the malleability and ductility of metals.
- Low density: They are generally less dense than metals.
- High electronegativity: They readily gain electrons to form negative ions.
- Lack metallic luster: They often appear dull or non-reflective.
The Definitive Case for Silver as a Metal
Silver, with its distinct properties, firmly falls under the metal classification. Let's examine its key characteristics to reinforce this categorization:
1. Exceptional Electrical Conductivity
Silver boasts the highest electrical conductivity of any element. This exceptional ability to conduct electricity is a hallmark of metals and stems from the readily available electrons in its outermost electron shell. These electrons can move freely within the silver's metallic lattice, facilitating the efficient flow of electrical current. This property is exploited in various applications, including electrical wiring, circuitry, and high-frequency applications.
2. High Thermal Conductivity
Similar to its electrical conductivity, silver also displays high thermal conductivity. This means it efficiently transfers heat energy. This property is crucial in applications like heat sinks and thermal management systems in electronics where heat dissipation is critical for optimal performance.
3. Malleability and Ductility
Silver is highly malleable (can be hammered into thin sheets) and ductile (can be drawn into wires). This ability to be easily shaped without breaking is a defining characteristic of metals. This contributes to its use in jewelry making, coinage, and other applications requiring intricate shapes and forms.
4. Metallic Luster and Appearance
Silver's bright, shiny appearance – its metallic luster – is another clear indicator of its metallic nature. This characteristic results from the interaction of light with its electron structure. The luster contributes to its aesthetic appeal and its use in decorative applications.
5. High Density
Silver has a relatively high density compared to nonmetals. This is a common trait of metals, stemming from the close packing of atoms in their crystal structures.
6. Low Ionization Energy
Silver's low ionization energy means it readily loses electrons to form positive ions. This ability to ionize is a fundamental property of metals and facilitates its participation in various chemical reactions and its formation of compounds.
Comparing Silver to Metalloids and Nonmetals
To further solidify silver's classification, let's explicitly compare it to metalloids and nonmetals:
Silver vs. Metalloids
Unlike metalloids, silver exhibits consistent and high electrical and thermal conductivity across a wide range of temperatures and conditions. Metalloids, such as silicon and germanium, demonstrate variable conductivity, often behaving as semiconductors, while silver consistently functions as an excellent conductor. The malleability and ductility of silver also sharply contrast with the brittle nature of most metalloids.
Silver vs. Nonmetals
The disparity between silver and nonmetals is even more pronounced. Nonmetals, such as sulfur and oxygen, are poor conductors of electricity and heat, exhibiting properties completely opposite to those of silver. Silver's malleability, ductility, and metallic luster are absent in nonmetals. Their chemical behavior also differs drastically; nonmetals tend to gain electrons to form anions, while silver readily loses electrons to form cations.
Applications Highlighting Silver's Metallic Properties
The widespread applications of silver directly stem from its remarkable metallic properties:
- Electrical Conductivity: Used extensively in electrical wiring, circuitry, and electronic components. Its high conductivity ensures efficient power transmission and minimal energy loss.
- Thermal Conductivity: Utilized in heat sinks and thermal management systems to dissipate heat and prevent overheating in electronic devices.
- Malleability and Ductility: Essential in jewelry making, coinage, and the creation of various decorative and functional items. Its ease of shaping allows for intricate designs and precise fabrication.
- Antimicrobial Properties: Silver nanoparticles exhibit potent antimicrobial properties, used in wound dressings, water purification systems, and various medical applications. This property, while related to its chemical reactivity, is a consequence of its metallic nature and surface area effects.
- Catalysis: Silver acts as a catalyst in various chemical reactions, its metallic properties influencing its catalytic activity.
- Photography: Historically crucial in photographic film, utilizing silver's light sensitivity.
- Mirrors: Silver's high reflectivity allows for the creation of high-quality mirrors.
Conclusion: Silver - An Unmistakable Metal
Based on its comprehensive set of properties, including its exceptional electrical and thermal conductivity, high malleability and ductility, metallic luster, high density, and low ionization energy, there is no ambiguity in classifying silver. Silver is undeniably a metal. Its unique combination of properties has led to a wide array of applications across various industries, emphasizing its crucial role in modern technology and society. The differences between silver and both metalloids and nonmetals are substantial, making the metal classification unambiguous and clearly supported by experimental evidence. Any assertion otherwise would be inaccurate and not supported by the scientific understanding of elemental properties.
Latest Posts
Latest Posts
-
Chapter 1 Biology The Study Of Life
Mar 29, 2025
-
Finding Critical Points Of Multivariable Functions
Mar 29, 2025
-
Why Is Frozen Water Less Dense Than Liquid Water
Mar 29, 2025
-
Predicting Products Of Chemical Reactions Practice Problems
Mar 29, 2025
-
Are Cells The Basic Unit Of Life
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Silver A Metal Metalloid Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
