Is Silver A Metal Nonmetal Or Metalloid
Muz Play
Mar 20, 2025 · 5 min read
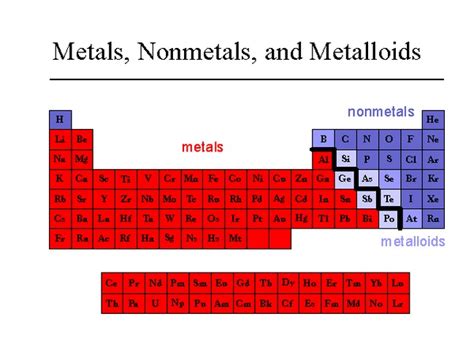
Table of Contents
Is Silver a Metal, Nonmetal, or Metalloid? A Comprehensive Exploration
Silver, a lustrous white metal known for its exceptional conductivity and malleability, is unequivocally classified as a metal. This article delves deep into the characteristics of silver, exploring its properties and comparing them to those of nonmetals and metalloids to definitively establish its metallic nature. We'll examine its physical and chemical properties, its position within the periodic table, and its widespread applications to solidify its classification as a metal.
Understanding the Classification of Elements
Before diving into the specifics of silver, it's crucial to understand the basic distinctions between metals, nonmetals, and metalloids. These classifications are based on several key properties:
-
Metals: Typically characterized by high electrical and thermal conductivity, malleability (ability to be hammered into sheets), ductility (ability to be drawn into wires), and metallic luster (shiny appearance). They tend to lose electrons easily, forming positive ions.
-
Nonmetals: Generally poor conductors of heat and electricity, brittle, and lack metallic luster. They often gain electrons to form negative ions.
-
Metalloids (Semimetals): Exhibit properties intermediate between metals and nonmetals. Their conductivity can vary depending on conditions, and they may display some metallic luster but lack the malleability and ductility of metals.
The Defining Properties of Silver: A Case for Metallicity
Silver's classification as a metal is undeniable due to its distinct physical and chemical properties:
Physical Properties:
-
Excellent Electrical Conductivity: Silver boasts the highest electrical conductivity of all metals, making it invaluable in electrical applications. This high conductivity stems from the ease with which electrons can move through its structure. This is a hallmark characteristic of metals.
-
Exceptional Thermal Conductivity: Similar to its electrical conductivity, silver exhibits outstanding thermal conductivity, efficiently transferring heat. This property is crucial in applications requiring efficient heat dissipation. Again, this aligns perfectly with metallic properties.
-
High Malleability and Ductility: Silver can be easily hammered into thin sheets (malleability) and drawn into wires (ductility), demonstrating its characteristic metallic nature. These properties are crucial for shaping silver into various forms for jewelry, coins, and other applications.
-
Metallic Luster: Silver possesses a brilliant, silvery-white luster, a defining characteristic of metals. This shine arises from the interaction of light with its delocalized electrons.
-
High Density: Silver is a relatively dense metal, meaning it has a large mass packed into a small volume. This is typical of many metals.
-
High Melting and Boiling Points: Silver has a relatively high melting point (962°C) and boiling point (2212°C) compared to nonmetals. Strong metallic bonding contributes to these high temperatures.
Chemical Properties:
-
Low Electronegativity: Silver has a low electronegativity, meaning it has a weak tendency to attract electrons. Metals generally have low electronegativity values, readily losing electrons in chemical reactions to form positive ions (cations).
-
Formation of Cations: Silver readily loses one electron to form a +1 ion (Ag⁺), a common characteristic of metals. This is evident in various silver compounds.
-
Reactivity: While not as reactive as some alkali metals, silver does react with certain substances, such as sulfur and halogens, forming compounds. This reactivity, though less pronounced than some other metals, still falls within the realm of metallic behavior.
-
Oxidation: Silver can undergo oxidation, reacting with oxygen in the air to form silver oxide (Ag₂O). Although this tarnish is a surface reaction, it's a testament to silver's interaction with its environment, albeit a relatively slow one. This is a chemical property consistent with metals, although the rate of oxidation varies considerably among different metals.
Comparing Silver to Nonmetals and Metalloids
To further emphasize silver's metallic classification, let's compare it to elements from the other two categories:
Silver vs. Nonmetals:
A stark contrast exists between silver and nonmetals. While silver exhibits excellent conductivity, high malleability, and ductility, nonmetals are generally poor conductors and brittle. Nonmetals, such as oxygen, chlorine, and sulfur, gain electrons to form negative ions (anions), unlike silver's tendency to lose electrons and form positive ions. Their physical appearances also differ significantly; nonmetals lack the metallic luster characteristic of silver.
Silver vs. Metalloids:
Metalloids like silicon and arsenic show properties intermediate between metals and nonmetals. While they can exhibit some metallic luster and have varying conductivity depending on conditions, they lack the high malleability and ductility that define silver. Furthermore, their chemical behavior tends to be more complex and less predictable than the straightforward cation formation seen in silver.
Silver's Position in the Periodic Table
Silver's location in the periodic table further supports its metallic classification. It is situated in Group 11, alongside copper and gold, all of which are transition metals. Transition metals are known for their characteristic metallic properties, including high conductivity and malleability. This consistent placement among metals in the periodic table provides additional evidence for its metallic nature.
Applications Highlighting Silver's Metallic Properties
Silver's unique combination of properties leads to its widespread use in diverse applications:
-
Electrical Conductivity: Silver's exceptional electrical conductivity makes it essential in electronics, including printed circuit boards, electrical contacts, and high-frequency applications.
-
Thermal Conductivity: In applications requiring efficient heat transfer, silver's high thermal conductivity is utilized in heat sinks and thermal pastes.
-
Malleability and Ductility: These properties allow silver to be easily shaped into intricate designs for jewelry and silverware. Its ability to be drawn into fine wires finds use in various industrial processes.
-
Catalysis: Silver's catalytic properties are exploited in various chemical reactions, notably in the production of ethylene oxide.
-
Photography: Silver halides have been historically important in photography due to their light sensitivity.
-
Medicine: Silver has antimicrobial properties, leading to its use in wound dressings and medical devices.
Conclusion: Silver is Undeniably a Metal
The evidence presented overwhelmingly supports the classification of silver as a metal. Its physical properties – high electrical and thermal conductivity, malleability, ductility, and metallic luster – coupled with its chemical properties – low electronegativity, formation of positive ions, and reactivity consistent with metals – leave no doubt. Its position in the periodic table, among other transition metals, and its widespread applications that leverage its metallic characteristics further solidify this classification. Any suggestion otherwise would contradict the well-established understanding of its properties and behavior. Silver's metallic nature is fundamental to its diverse applications and its crucial role in various industries and technologies.
Latest Posts
Latest Posts
-
Is A Circle On A Graph A Function
Mar 20, 2025
-
How To Assign D And L Configuration
Mar 20, 2025
-
How To Use Inverse Matrices To Solve System Of Equations
Mar 20, 2025
-
What Does The Activity Theory State
Mar 20, 2025
-
What Is A Mixed Melting Point
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Silver A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
