How To Assign D- And L-configuration
Muz Play
Mar 20, 2025 · 6 min read
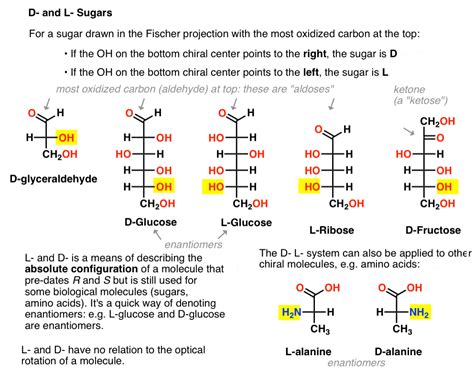
Table of Contents
How to Assign D- and L-Configuration: A Comprehensive Guide
Assigning D- and L-configurations to chiral molecules is a fundamental concept in organic chemistry. Understanding this system is crucial for accurately representing the three-dimensional structure of molecules and predicting their properties and interactions. While seemingly complex at first, mastering D/L nomenclature becomes significantly easier with a systematic approach. This comprehensive guide will break down the process step-by-step, providing you with the tools and knowledge to confidently assign D- and L-configurations.
Understanding Chirality and Stereoisomers
Before diving into D/L configuration, let's clarify the underlying concepts. Chirality refers to a molecule's property of being non-superimposable on its mirror image. Molecules exhibiting chirality are called chiral molecules. This lack of superimposition is due to the presence of one or more chiral centers, typically a carbon atom bonded to four different groups. Chiral molecules exist as stereoisomers, which are molecules with the same molecular formula and connectivity but different spatial arrangements of atoms. Enantiomers are a specific type of stereoisomer that are non-superimposable mirror images of each other. Diastereomers, on the other hand, are stereoisomers that are not mirror images.
The D/L system, also known as the relative configuration system, is a way of designating the absolute configuration of a chiral molecule relative to glyceraldehyde. This is historically significant and remains widely used, particularly in biochemistry. It’s important to distinguish this from the R/S system, which is an absolute configuration system based on the Cahn-Ingold-Prelog (CIP) priority rules. While the R/S system is now preferred for its unambiguous nature, understanding the D/L system is still essential.
The D/L System: A Historical Perspective
The D/L system is rooted in the historical context of carbohydrate chemistry. The system's origins trace back to the work of Emil Fischer who used the naturally occurring isomers of glyceraldehyde as a reference point. Glyceraldehyde, the simplest aldotriose, possesses one chiral center and thus exists as two enantiomers. Fischer arbitrarily assigned the configuration of one enantiomer as D and the other as L.
This arbitrary assignment, based on the orientation of the hydroxyl group (-OH) at the chiral center, was a crucial starting point. The D-glyceraldehyde was assigned to the isomer where the hydroxyl group is on the right when the molecule is drawn with the aldehyde group at the top and the carbon chain extending downwards (Fischer projection). Conversely, L-glyceraldehyde is assigned to the isomer with the hydroxyl group on the left.
Assigning D and L Configurations: A Step-by-Step Guide
The D/L system is applied to other chiral molecules by comparing their structures to glyceraldehyde. The following steps outline the process:
-
Identify the Chiral Center: The first step involves identifying the chiral center within the molecule. This is typically a carbon atom bonded to four different groups.
-
Draw the Fischer Projection: Represent the molecule in a Fischer projection. This is a two-dimensional representation where the molecule is drawn with the carbon chain vertically, and substituents are projected horizontally or vertically. Ensure the longest carbon chain is oriented vertically.
-
Find the Highest Numbered Chiral Center: In molecules with multiple chiral centers, focus on the highest-numbered chiral center along the main carbon chain.
-
Orient the Molecule: Orient the molecule to make it structurally resemble glyceraldehyde. Focus on the carbon chain and the position of relevant functional groups.
-
Compare the Hydroxyl Group: Observe the position of the hydroxyl group (-OH) or a similar group at the highest-numbered chiral center.
-
Assign D or L: If the hydroxyl group (or corresponding group) is on the right in the Fischer projection, the molecule is designated as D. If it's on the left, the molecule is designated as L.
Important Note: The D/L system solely focuses on the relative configuration concerning glyceraldehyde. It does not provide information about the absolute configuration (R or S). Moreover, molecules with multiple chiral centers require assigning D or L to each individual chiral center separately.
Example: Assigning D and L Configuration to a Sugar
Let's illustrate the process with an example: consider the sugar molecule, D-fructose.
-
Identify the Chiral Center: D-Fructose has multiple chiral centers. We focus on the highest-numbered chiral center in the main chain.
-
Draw the Fischer Projection: A Fischer projection of D-Fructose is drawn with the ketone group (C=O) at the top.
-
Orient the Molecule: We orient the molecule such that the carbon chain and other substituents structurally align with the model we established for glyceraldehyde.
-
Compare the Hydroxyl Group: The hydroxyl group on the highest-numbered chiral center is to the right.
-
Assign D or L: This leads to the assignment of D-fructose.
Practical Applications of D/L Nomenclature
The D/L system holds significant relevance in various fields, particularly in biochemistry and pharmacology:
-
Carbohydrate Chemistry: The system is fundamental in carbohydrate chemistry, providing a convenient way to classify sugars and their isomers. Many biologically important carbohydrates, like glucose and fructose, are defined using the D/L nomenclature.
-
Amino Acid Chemistry: Amino acids, the building blocks of proteins, are also classified using the D/L system, though the reference point is not glyceraldehyde but rather L-glyceraldehyde. L-amino acids are prevalent in proteins, whereas D-amino acids are less common. This distinction is vital in understanding protein structure and function.
-
Pharmaceutical Chemistry: The D/L configuration of a molecule can significantly influence its biological activity and its interaction with receptors in the body. Enantiomers may have different effects – one could be therapeutically active, while the other is inactive or even toxic. This is a critical aspect in drug development and design.
Limitations of the D/L System
While historically significant, the D/L system has some limitations:
-
Ambiguity: The system relies on relative configuration and requires comparing molecules to glyceraldehyde, which can be ambiguous in some cases.
-
Inflexibility: It cannot encompass all types of chiral molecules.
-
Multiple Chiral Centers: When multiple chiral centers are present, assigning D/L for each individual center can become tedious.
Transitioning to the R/S System
The R/S system, based on the Cahn-Ingold-Prelog (CIP) priority rules, provides an unambiguous way to assign absolute configuration, addressing the shortcomings of the D/L system. While the D/L system remains relevant historically and in specific contexts, the R/S system is now the preferred method for assigning absolute configuration due to its clarity and wider applicability. Learning both systems is highly beneficial for a complete understanding of stereochemistry.
Conclusion: Mastering D/L and R/S Configurations
The assignment of D- and L-configurations, though initially appearing challenging, becomes manageable with systematic practice. Understanding the underlying principles of chirality and stereoisomers is crucial. By following the step-by-step guide provided, you can confidently apply the D/L nomenclature to various molecules. However, it is essential to acknowledge the limitations of the D/L system and appreciate the superiority of the R/S system in unambiguous absolute configuration assignment. Combining the knowledge of both systems will enhance your understanding of stereochemistry and its significance in various fields, from biochemistry to pharmacology. Through practice and familiarity with these methods, assigning configurations will become second nature, enriching your study of organic chemistry and its related disciplines.
Latest Posts
Latest Posts
-
What Element Has 8 Protons And 8 Neutrons
Mar 20, 2025
-
The Law Of Sines Ambiguous Case
Mar 20, 2025
-
How To Find The Instantaneous Acceleration
Mar 20, 2025
-
Periodic Table Gases Liquids And Solids
Mar 20, 2025
-
The Dorsal Body Cavity Is Subdivided Into The
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How To Assign D- And L-configuration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
