Periodic Table Gases Liquids And Solids
Muz Play
Mar 20, 2025 · 6 min read
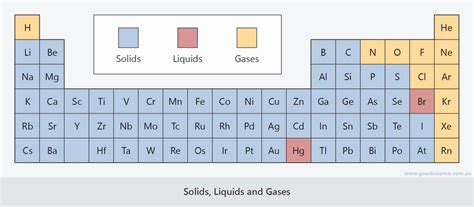
Table of Contents
Periodic Table: Gases, Liquids, and Solids – A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While we often focus on the table's overall structure and the trends in electronegativity, ionization energy, and atomic radius, a fascinating aspect lies in understanding the states of matter of these elements at standard temperature and pressure (STP). This article delves into the fascinating world of elements as gases, liquids, and solids, exploring their properties, distributions across the table, and the factors influencing their physical states.
Understanding States of Matter
Before diving into specific elements, let's refresh our understanding of the three primary states of matter:
1. Solids: Solids possess a definite shape and volume. Their constituent particles (atoms, ions, or molecules) are tightly packed in a regular, ordered arrangement, held together by strong intermolecular forces. This rigid structure resists compression and deformation. Examples include iron (Fe), diamond (C), and sodium chloride (NaCl).
2. Liquids: Liquids have a definite volume but take the shape of their container. Particles in liquids are closer together than in gases but are not as rigidly ordered as in solids. They exhibit some degree of freedom to move past one another, leading to fluidity. Examples include water (H₂O), mercury (Hg), and bromine (Br₂).
3. Gases: Gases have neither a definite shape nor volume. Their particles are widely dispersed and move randomly at high speeds, resulting in compressibility and the ability to fill any container completely. Weak intermolecular forces exist between gas particles. Examples include oxygen (O₂), nitrogen (N₂), and helium (He).
Gases on the Periodic Table
At STP, a significant number of elements exist as gases. Most are nonmetals located on the right side of the periodic table. Their low boiling points and weak intermolecular forces contribute to their gaseous nature.
Noble Gases (Group 18): The Unreactive Gases
The noble gases – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – are all gases at STP. Their exceptional stability due to their full valence electron shells leads to minimal interatomic interactions, resulting in low boiling points and inertness. Helium, in particular, is exceptionally light and has unique applications in cryogenics and lifting gases.
Diatomic Gases (Groups 14-17): Bonding and Gaseous State
Several elements exist as diatomic molecules (two atoms bonded together) in the gaseous phase at STP. These include:
-
Hydrogen (H₂): The lightest element, hydrogen forms a diatomic molecule through a covalent bond. It's crucial for numerous industrial processes and is a potential fuel source.
-
Nitrogen (N₂): A major component of Earth's atmosphere, nitrogen also forms a strong triple bond in its diatomic form. It's inert at standard temperatures but plays vital roles in biological processes and industrial fertilizer production.
-
Oxygen (O₂): Essential for respiration and combustion, oxygen exists as a diatomic molecule with a double bond. It is highly reactive and crucial for numerous chemical reactions.
-
Fluorine (F₂): The most reactive nonmetal, fluorine exists as a diatomic gas with a single bond. It's highly toxic and used in industrial applications.
-
Chlorine (Cl₂): A halogen, chlorine exists as a diatomic gas with a single bond. It's used as a disinfectant and in various industrial processes.
These diatomic gases exhibit relatively weak intermolecular forces compared to their solid or liquid counterparts, allowing them to exist as gases at STP.
Liquids on the Periodic Table
Relatively few elements exist as liquids at STP. Their position on the periodic table and their intermolecular forces play crucial roles.
Mercury (Hg): The Only Metallic Liquid
Mercury, a heavy metal in Group 12, is the only element that is liquid at standard temperature and pressure. Its unique electronic structure and weak metallic bonding contribute to its low melting point and liquid state. It is historically significant but highly toxic and should be handled with extreme caution.
Bromine (Br₂): The Only Non-Metallic Liquid
Bromine, a halogen in Group 17, is the only non-metal liquid at STP. While it forms diatomic molecules like chlorine and fluorine, its larger atomic size and increased intermolecular forces lead to a higher boiling point, resulting in a liquid state at STP. Bromine is a strong oxidizing agent with various industrial applications.
Solids on the Periodic Table
The vast majority of elements in the periodic table exist as solids at STP. Their strong interatomic or intermolecular forces hold their atoms tightly together in a structured lattice.
Metals: The Backbone of Solids
Metals dominate the left side and center of the periodic table. Their strong metallic bonding, involving the delocalization of valence electrons, leads to high melting points, good electrical conductivity, and malleability. Examples include iron (Fe), copper (Cu), gold (Au), and aluminum (Al). Many metals exhibit crystalline structures with varying degrees of hardness and ductility.
Non-metals: Diverse Solid Forms
Non-metallic elements exhibit a wider range of solid forms, depending on their bonding and structure. Some form network covalent solids like diamond (C) and silicon dioxide (SiO₂), which have incredibly high melting points due to their strong covalent bonds. Others form molecular solids, where individual molecules are held together by weaker intermolecular forces, resulting in lower melting points. Examples include iodine (I₂), phosphorus (P₄), and sulfur (S₈).
Factors Affecting the State of Matter
Several factors influence whether an element exists as a solid, liquid, or gas at STP:
1. Atomic/Molecular Size and Mass: Larger atoms and molecules generally have stronger London dispersion forces, leading to higher melting and boiling points and a greater likelihood of existing as solids or liquids.
2. Intermolecular Forces: Stronger intermolecular forces (hydrogen bonding, dipole-dipole interactions, London dispersion forces) lead to higher melting and boiling points. Noble gases, with only weak London dispersion forces, exist as gases at STP, while elements with strong hydrogen bonding, like water, exist as liquids.
3. Bonding Type: The type of bonding significantly impacts the state of matter. Metallic bonding in metals results in high melting points and solid states, while covalent bonding in network solids like diamond leads to extremely high melting points, also resulting in a solid state. Weak Van der Waals forces in noble gases result in gaseous states.
4. Temperature and Pressure: Changing temperature and pressure can alter the state of matter. Increasing temperature generally favors the gaseous state, while increasing pressure favors the liquid or solid state. Phase diagrams illustrate the relationships between temperature, pressure, and the state of matter.
Conclusion: The Periodic Table and States of Matter
The periodic table provides a powerful framework for understanding the properties of elements, including their states of matter at STP. The systematic arrangement reveals trends in atomic size, intermolecular forces, and bonding types that influence whether an element exists as a gas, liquid, or solid. While the majority of elements are solids at standard conditions, understanding the factors that contribute to the gaseous and liquid states of certain elements helps us comprehend the diverse properties and behaviors of matter in the world around us. This comprehensive understanding is crucial in various scientific and technological fields, ranging from materials science and engineering to atmospheric science and environmental chemistry. Further exploration of phase diagrams and critical points offers a deeper insight into the dynamic interactions between temperature, pressure, and the states of matter for individual elements and compounds.
Latest Posts
Latest Posts
-
What Color Is The Frog Galbladder
Mar 21, 2025
-
Write The Inequality In Interval Notation
Mar 21, 2025
-
When Do We Use Prefixes In Naming Compounds
Mar 21, 2025
-
Photosynthesis In C4 And Cam Plants
Mar 21, 2025
-
What Is A Compound Light Microscope
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Gases Liquids And Solids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
