When Do We Use Prefixes In Naming Compounds
Muz Play
Mar 21, 2025 · 5 min read
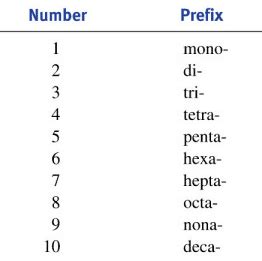
Table of Contents
When Do We Use Prefixes in Naming Compounds? A Comprehensive Guide
Understanding when to use prefixes in naming chemical compounds is crucial for clear communication and accurate representation in chemistry. This guide delves deep into the rules and exceptions governing prefix usage, providing a comprehensive overview for students and professionals alike. We will explore the systematic approach to nomenclature, focusing on binary compounds, ionic compounds, and covalent compounds.
The Importance of Prefixes in Chemical Nomenclature
Chemical nomenclature, the system of naming chemical compounds, relies heavily on prefixes to unambiguously identify the composition of a substance. Without prefixes, particularly in covalent compounds, the formula would be ambiguous, leading to potential misunderstandings and errors. The systematic use of prefixes ensures that the name of a compound precisely reflects its chemical formula and vice-versa. This is particularly important in fields like pharmaceuticals, materials science, and analytical chemistry where precision is paramount.
Prefixes for Covalent Compounds: A Detailed Explanation
Covalent compounds are formed by the sharing of electrons between non-metal atoms. In naming these compounds, prefixes are essential to indicate the number of atoms of each element present in the molecule. The most common prefixes are:
- Mono- (1): Used only for the second element in the name if there's more than one atom. Often omitted for the first element.
- Di- (2): Indicates two atoms of the element.
- Tri- (3): Indicates three atoms of the element.
- Tetra- (4): Indicates four atoms of the element.
- Penta- (5): Indicates five atoms of the element.
- Hexa- (6): Indicates six atoms of the element.
- Hepta- (7): Indicates seven atoms of the element.
- Octa- (8): Indicates eight atoms of the element.
- Nona- (9): Indicates nine atoms of the element.
- Deca- (10): Indicates ten atoms of the element.
Examples of Prefix Usage in Covalent Compounds:
- CO: Carbon monoxide (The mono- prefix is used only for the second element).
- CO₂: Carbon dioxide
- N₂O₄: Dinitrogen tetroxide
- PCl₅: Phosphorus pentachloride
- SF₆: Sulfur hexafluoride
- N₂O₅: Dinitrogen pentoxide
- As₂O₅: Diarsenic pentoxide
Exceptions and Special Cases in Covalent Compounds:
While the rules are generally straightforward, there are exceptions:
- Common Names: Some covalent compounds have common names that don't follow the standard prefix rules (e.g., water (H₂O) and ammonia (NH₃)).
- Acids: Acids have their own naming conventions and do not always follow the standard prefix rules for covalent compounds.
Prefixes in Ionic Compounds: A Closer Look
Ionic compounds are formed by the electrostatic attraction between oppositely charged ions (cations and anions). Prefixes are generally not used in naming simple ionic compounds. Instead, the names are derived from the names of the constituent ions, using Roman numerals to indicate the charge of the cation when necessary (particularly with transition metals that can have multiple oxidation states).
Examples of Ionic Compound Naming:
- NaCl: Sodium chloride (no prefixes needed)
- MgO: Magnesium oxide (no prefixes needed)
- FeCl₂: Iron(II) chloride (Roman numeral indicates the 2+ charge of iron)
- FeCl₃: Iron(III) chloride (Roman numeral indicates the 3+ charge of iron)
- CuSO₄: Copper(II) sulfate (Roman numeral indicates the 2+ charge of copper)
Polyatomic Ions and Prefixes:
While prefixes are typically not used for simple ionic compounds, they might be used in compounds containing polyatomic ions with variable numbers. However, the use of prefixes in this context is less common and often depends on the context and convention. For instance, in naming compounds with different numbers of a polyatomic anion, the prefixes can be used for clarity (e.g. disodium phosphate).
Prefixes in Other Chemical Naming Systems
The use of prefixes extends beyond simple binary and ionic compounds. They play a role in various other chemical naming systems, including:
- Organic Chemistry: Prefixes are extensively used in organic chemistry to denote the number of carbon atoms in a chain or ring (e.g., meth-, eth-, prop-, but-). They also indicate the position and type of substituents.
- Coordination Compounds: Coordination compounds utilize prefixes to specify the number of ligands attached to a central metal atom.
Practical Application and Problem Solving
Understanding prefix usage is crucial for accurately translating between chemical names and formulas. Let's work through some examples to solidify this understanding:
Problem 1: What is the name of the compound with the formula P₄O₁₀?
Solution: Following the rules for covalent compounds, we use prefixes to represent the number of atoms: Tetra- for four phosphorus atoms and deca- for ten oxygen atoms. Therefore, the name is tetraphosphorus decoxide.
Problem 2: What is the chemical formula for silicon tetrafluoride?
Solution: The name indicates one silicon atom (no prefix for the first element) and four fluoride atoms (tetra-). Therefore, the chemical formula is SiF₄.
Problem 3: What is the name of Fe₂O₃?
Solution: This is an ionic compound. Iron (Fe) is a transition metal with variable oxidation states. To determine the oxidation state of Iron, we consider the charge of the Oxygen atoms, which are -2 each. Therefore, the overall charge of the oxygen atoms is -6. The total charge must be zero. To balance this -6 charge, two Iron atoms must have a total charge of +6. Hence each iron atom has a +3 charge. Therefore, the name is Iron(III) oxide.
Conclusion: Mastering Prefix Usage in Chemical Nomenclature
Mastering the use of prefixes in chemical nomenclature is essential for accurate communication in chemistry. While the basic rules are straightforward, understanding the exceptions and nuances is crucial for proficiency. By carefully considering the type of compound (covalent or ionic), the elements involved, and the relevant conventions, you can accurately name and interpret chemical formulas, ensuring clear and unambiguous communication within the scientific community. Consistent practice with various examples will help build confidence and expertise in this fundamental aspect of chemistry. Remember to consult reliable chemistry textbooks and resources for further detailed information and clarification on specific cases.
Latest Posts
Latest Posts
-
Rusting Of Iron Chemical Or Physical Change
Mar 27, 2025
-
How Many Neutrons Does K Have
Mar 27, 2025
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
-
Song Lyrics Ode To Billy Joe
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about When Do We Use Prefixes In Naming Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
