What Element Has 8 Protons And 8 Neutrons
Muz Play
Mar 20, 2025 · 6 min read
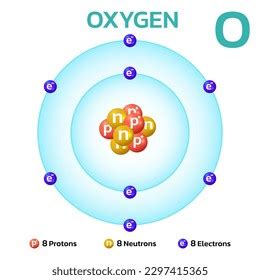
Table of Contents
What Element Has 8 Protons and 8 Neutrons? Unveiling the Mystery of Oxygen
The question, "What element has 8 protons and 8 neutrons?" leads us on a fascinating journey into the heart of atomic structure and the periodic table. The answer, as we'll discover, is oxygen, a vital element for life as we know it. This article will delve deep into the properties of oxygen, its isotopic variations, its significance in biological processes, and its broader industrial applications. We'll explore the concepts of atomic number, mass number, and isotopes to fully understand the unique characteristics of this element with 8 protons and 8 neutrons.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the element with 8 protons and 8 neutrons, let's establish a foundational understanding of atomic structure. An atom, the fundamental building block of matter, comprises three subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines an element's atomic number and its unique identity on the periodic table.
- Neutrons: Neutrally charged particles also located in the nucleus. They contribute to the atom's mass but not its charge. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom.
The atomic number, represented by 'Z', is the number of protons in an atom's nucleus. The mass number, represented by 'A', is the total number of protons and neutrons in the nucleus. Therefore, A = Z + N, where N represents the number of neutrons.
Identifying the Element: Oxygen (¹⁶O)
An element with 8 protons has an atomic number of 8. Consulting the periodic table, we find that element number 8 is oxygen (O). The question specifies 8 neutrons. Therefore, the mass number (A) is 8 (protons) + 8 (neutrons) = 16. This specific isotope of oxygen is represented as ¹⁶O, where the superscript 16 denotes the mass number.
This particular isotope, ¹⁶O, is the most abundant and stable isotope of oxygen, comprising about 99.76% of all naturally occurring oxygen.
Isotopes of Oxygen: Variations in Neutron Number
While the most common oxygen isotope has 8 neutrons, oxygen exists in other isotopic forms with varying numbers of neutrons. These are called isotopes, which are atoms of the same element with the same number of protons but different numbers of neutrons. Some common oxygen isotopes include:
- ¹⁶O (Oxygen-16): 8 protons, 8 neutrons – the most abundant and stable isotope.
- ¹⁷O (Oxygen-17): 8 protons, 9 neutrons – a stable but less abundant isotope.
- ¹⁸O (Oxygen-18): 8 protons, 10 neutrons – a stable but less abundant isotope.
These different isotopes have slightly different masses due to the varying neutron counts, but their chemical properties remain essentially the same because the number of protons (and hence electrons) determines chemical behavior. However, the differences in mass can be exploited in various scientific applications, such as isotopic tracing in biological and geological studies.
The Importance of Oxygen in Biological Systems
Oxygen plays a crucial role in sustaining life on Earth. Its significance stems from its involvement in cellular respiration, the process by which living organisms convert nutrients into energy. In aerobic respiration, oxygen acts as the final electron acceptor in the electron transport chain, a series of reactions that generate ATP, the primary energy currency of cells. Without oxygen, this efficient energy production pathway would be unavailable, drastically limiting life's capabilities.
Oxygen's involvement extends beyond energy production. It's a vital component of water (H₂O), a fundamental solvent for biological processes and a crucial component of many biological molecules. Furthermore, oxygen is a constituent of many essential organic molecules, including carbohydrates, lipids, proteins, and nucleic acids.
Oxygen's Industrial Applications: A Versatile Element
Beyond its biological importance, oxygen finds widespread use in various industrial applications. Its high reactivity makes it crucial in many chemical processes:
- Steelmaking: Oxygen is used in the basic oxygen furnace (BOF) process to remove impurities from molten iron, producing high-quality steel.
- Welding and Cutting: Oxygen-fuelled torches provide high temperatures for welding and cutting metals.
- Chemical Synthesis: Oxygen serves as a reactant in various chemical syntheses, producing numerous important compounds.
- Water Treatment: Oxygen is employed in wastewater treatment to promote the breakdown of organic matter by aerobic bacteria.
- Medicine: Oxygen therapy is used to treat respiratory conditions where oxygen levels in the blood are low.
Environmental Significance of Oxygen: A Delicate Balance
Maintaining adequate levels of atmospheric oxygen is crucial for the health of the planet and its inhabitants. Photosynthesis, the process by which plants and other photosynthetic organisms convert light energy into chemical energy, is responsible for replenishing atmospheric oxygen. The balance between oxygen production and consumption is a delicate one, and any significant disruption can have profound consequences.
The increasing levels of greenhouse gases, such as carbon dioxide, are causing changes to the global climate, impacting oxygen levels indirectly. Deforestation and pollution also contribute to imbalances in the atmospheric composition. Understanding and preserving the delicate balance of oxygen in the environment is crucial for safeguarding the health of the planet and all its life forms.
Oxygen Isotopes and Scientific Applications
The different isotopes of oxygen, particularly ¹⁸O, are valuable tools in various scientific fields. Their mass differences allow scientists to track the movement of oxygen atoms through different systems. This technique, known as isotopic tracing, has numerous applications:
- Paleoclimatology: Analyzing the ratio of ¹⁸O to ¹⁶O in ice cores and marine sediments helps to reconstruct past climates.
- Hydrology: Tracking the flow of water in rivers and aquifers.
- Biology: Studying metabolic processes and tracing the movement of oxygen in biological systems.
- Archaeology: Dating ancient artifacts and understanding past environments.
The subtle differences in mass between oxygen isotopes are exploited in these applications, providing invaluable insights into various natural processes.
Conclusion: Oxygen - A Ubiquitous and Essential Element
In conclusion, the element with 8 protons and 8 neutrons is oxygen (¹⁶O), the most abundant and stable isotope of this vital element. Oxygen's importance extends beyond its presence in the air we breathe. It's a fundamental component of water, essential organic molecules, and plays a pivotal role in numerous biological processes and industrial applications. Understanding its atomic structure, isotopic variations, and its multifaceted roles is essential for appreciating its significance in various aspects of science, technology, and life itself. The careful monitoring and preservation of oxygen levels in our environment are crucial for maintaining the delicate balance that sustains life on Earth. The continued exploration and understanding of oxygen and its isotopes will continue to yield valuable insights into the complexities of our world.
Latest Posts
Latest Posts
-
Heat Transfer Throught The Collision Of Moluces Direct Contacr
Mar 21, 2025
-
What Color Is The Frog Galbladder
Mar 21, 2025
-
Write The Inequality In Interval Notation
Mar 21, 2025
-
When Do We Use Prefixes In Naming Compounds
Mar 21, 2025
-
Photosynthesis In C4 And Cam Plants
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Element Has 8 Protons And 8 Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
