Is State Of Matter A Physical Change/
Muz Play
Mar 16, 2025 · 6 min read
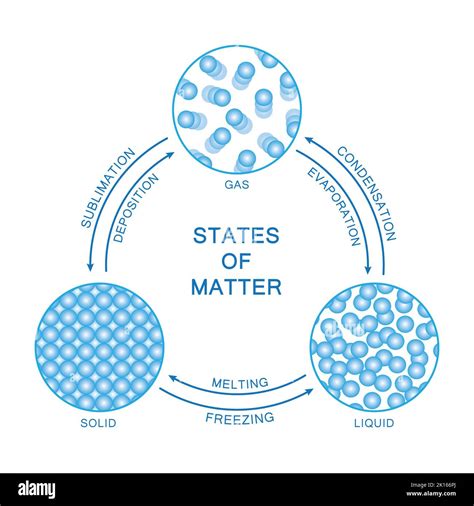
Table of Contents
Is a Change of State of Matter a Physical Change? A Deep Dive
The question of whether a change of state of matter is a physical change or a chemical change is a fundamental concept in science, often encountered early in chemistry education. The short answer is: yes, a change of state is a physical change. But understanding why requires a deeper look into the nature of matter, physical changes, and chemical changes. This article will explore this topic thoroughly, examining the underlying principles and providing illustrative examples.
Understanding the States of Matter
Before delving into the changes, let's establish a solid foundation by reviewing the common states of matter: solid, liquid, gas, and plasma. These states are distinguished by the arrangement and interaction of their constituent particles (atoms, molecules, or ions).
Solids
In solids, particles are tightly packed in a fixed, ordered arrangement. This strong intermolecular force restricts their movement, resulting in a definite shape and volume. Think of the rigid structure of a crystal or the solid form of ice.
Liquids
Liquids have a less ordered structure than solids. Particles are still relatively close together, but they can move and slide past each other. This explains why liquids flow and take the shape of their container, while maintaining a relatively constant volume. Water is a classic example.
Gases
In gases, particles are far apart and move randomly at high speeds. The weak intermolecular forces allow gases to expand to fill any container, exhibiting neither a definite shape nor volume. Air is a mixture of various gases.
Plasma
Plasma is often considered the fourth state of matter. It's an ionized gas, meaning the atoms have lost or gained electrons, resulting in charged particles. Plasma is found in stars, lightning, and fluorescent lights.
The Difference Between Physical and Chemical Changes
The core of our question hinges on differentiating physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The fundamental building blocks of the substance remain the same; only the arrangement or state of those blocks is altered. Examples include:
- Changes in state: Melting, freezing, boiling, condensation, sublimation (solid to gas), and deposition (gas to solid).
- Changes in shape: Cutting, bending, crushing.
- Dissolving: Salt dissolving in water. The salt is still salt; it's just dispersed in the water.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the formation of new substances with different chemical properties. This is characterized by the breaking and forming of chemical bonds, resulting in a fundamental alteration of the substance's composition. Examples include:
- Burning: Wood burning in air produces ashes, smoke, and gases.
- Rusting: Iron reacting with oxygen to form iron oxide (rust).
- Cooking: Many cooking processes involve chemical reactions that change the properties of food.
Why Changes of State are Physical Changes
Now, we can definitively state that changes of state are physical changes because they do not alter the chemical composition of the substance.
Consider water as an example. When ice (solid water) melts into liquid water, or liquid water boils into steam (gaseous water), the molecules remain H₂O. The only change is the arrangement and energy of the water molecules. In ice, the molecules are tightly bound in a crystal lattice. In liquid water, they move more freely. In steam, they move very rapidly and independently. No new substance is formed. The chemical bonds within the water molecule remain intact throughout the process.
This same principle applies to other substances. When solid iodine sublimes into gaseous iodine, the iodine molecules remain iodine molecules; they simply transition from a solid to a gaseous state. Similarly, the freezing of liquid mercury into solid mercury does not alter the composition of mercury atoms.
Deeper Look into the Molecular Level
Understanding the molecular level provides a more profound appreciation for why changes of state are physical. The forces between molecules (intermolecular forces) are crucial. These forces are relatively weak compared to the intramolecular forces (the bonds within a molecule). Changes of state primarily involve overcoming or modifying intermolecular forces. For instance:
- Melting: Adding energy (heat) overcomes the intermolecular forces holding the solid structure together, allowing the molecules to move more freely.
- Boiling: Further addition of energy provides enough kinetic energy for molecules to completely overcome intermolecular forces and escape into the gaseous phase.
- Freezing: Removing energy (heat) weakens the kinetic energy of the molecules, allowing intermolecular forces to draw them closer and form a more ordered solid structure.
In contrast, chemical changes involve breaking and forming chemical bonds—the strong intramolecular forces within a molecule. This requires significantly more energy than simply changing the state of matter.
Examples of Changes of State as Physical Changes
Let's examine several specific examples to further solidify the concept:
Water: The quintessential example
- Ice melting: Ice (H₂O(s)) melts into liquid water (H₂O(l)). The chemical formula remains unchanged.
- Water boiling: Liquid water (H₂O(l)) boils into steam (H₂O(g)). Again, no change in chemical composition.
- Water freezing: Liquid water (H₂O(l)) freezes into ice (H₂O(s)). The molecules are simply rearranging themselves.
Other Substances
- Dry ice sublimation: Solid carbon dioxide (CO₂(s)) directly transforms into gaseous carbon dioxide (CO₂(g)) without an intermediate liquid phase. The molecule remains CO₂.
- Naphthalene sublimation: Naphthalene (mothballs) undergoes sublimation, transitioning directly from a solid to a gas. The chemical composition remains constant.
- Mercury freezing: Liquid mercury (Hg(l)) freezes into solid mercury (Hg(s)). The mercury atoms remain mercury atoms.
Distinguishing Physical and Chemical Changes: A Practical Approach
It is important to note that while changes of state are unequivocally physical changes, some processes might appear to involve both physical and chemical changes. For example, consider the electrolysis of water. While the water undergoes a physical change of state (liquid to gas), the process itself involves a chemical reaction (decomposition of water into hydrogen and oxygen). It's important to carefully analyze the nature of the changes to determine if it's purely physical or if there's a chemical component involved.
To determine whether a change is physical or chemical, consider the following:
- Is the chemical composition changed? If a new substance is formed with different chemical properties, it's a chemical change.
- Can the change be reversed easily? Physical changes are often easily reversible (e.g., melting ice and freezing water). Chemical changes are often irreversible (e.g., burning wood).
- Are there signs of a chemical reaction? Look for evidence such as gas production, color change, temperature change, or precipitate formation. These are often indicators of a chemical change.
Conclusion: The unequivocal nature of phase transitions
In conclusion, a change of state of matter is definitively a physical change. This is because the chemical composition of the substance remains unchanged throughout the process. The changes observed are due to alterations in the arrangement and energy of the molecules, reflecting changes in intermolecular forces but not intramolecular forces. Understanding this fundamental principle is crucial for grasping the basic concepts of matter and its transformations. Careful observation and analysis are necessary to distinguish between purely physical changes and those involving both physical and chemical transformations. The examples provided illustrate the clear distinction between these two types of changes, helping solidify the understanding of this crucial scientific concept.
Latest Posts
Latest Posts
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is State Of Matter A Physical Change/ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
