Is Turnover Number Affected By Substrate Concentration
Muz Play
Mar 24, 2025 · 5 min read
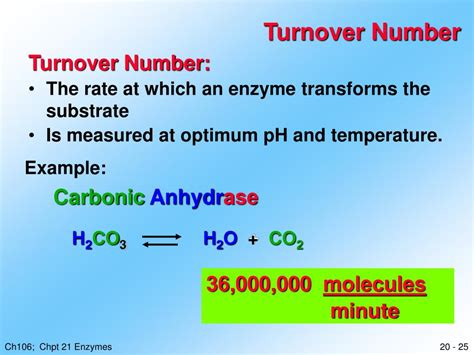
Table of Contents
Is Turnover Number Affected by Substrate Concentration?
Turnover number (k<sub>cat</sub>), also known as the catalytic constant, is a crucial kinetic parameter that reflects the maximum number of substrate molecules an enzyme can convert into product per unit time when the enzyme is saturated with substrate. Understanding how substrate concentration influences this number is vital for comprehending enzyme kinetics and designing efficient enzymatic processes. While the theoretical turnover number remains constant regardless of substrate concentration, the observed turnover number can be significantly affected by several factors intricately linked to substrate availability. Let's delve into the complexities of this relationship.
Understanding Turnover Number (k<sub>cat</sub>)
Before exploring the influence of substrate concentration, it's crucial to establish a clear understanding of k<sub>cat</sub> itself. It represents the rate constant of the reaction when the enzyme is completely saturated with substrate – meaning every active site is occupied. This saturation ensures that the rate-limiting step isn't the binding of the substrate but rather the intrinsic catalytic process itself. The equation for k<sub>cat</sub> is:
k<sub>cat</sub> = V<sub>max</sub> / [E]<sub>T</sub>
where:
- V<sub>max</sub> is the maximum reaction velocity, the rate at which the reaction proceeds when the enzyme is saturated with substrate.
- [E]<sub>T</sub> is the total enzyme concentration.
Essentially, k<sub>cat</sub> provides a measure of the enzyme's catalytic efficiency under optimal conditions. A higher k<sub>cat</sub> indicates a faster and more efficient enzyme.
The Michaelis-Menten Equation and Substrate Concentration
The Michaelis-Menten equation is fundamental to understanding enzyme kinetics and the relationship between substrate concentration and reaction velocity:
v = (V<sub>max</sub>[S]) / (K<sub>m</sub> + [S])
where:
- v is the initial reaction velocity.
- [S] is the substrate concentration.
- K<sub>m</sub> is the Michaelis constant, representing the substrate concentration at which the reaction velocity is half of V<sub>max</sub>.
This equation reveals a crucial aspect of enzyme behavior: at low substrate concentrations, the reaction velocity increases proportionally to the substrate concentration. However, as substrate concentration increases, the velocity plateaus, approaching V<sub>max</sub>. This saturation effect is due to all enzyme active sites becoming occupied.
How Substrate Concentration Appears to Affect Turnover Number
While the theoretical k<sub>cat</sub> remains constant, the observed turnover number can seem to vary with substrate concentration, primarily due to these factors:
1. Substrate Inhibition:
At very high substrate concentrations, substrate inhibition can occur. This phenomenon arises when excess substrate molecules bind to sites other than the active site, altering the enzyme's conformation and reducing its catalytic activity. This leads to a decrease in the observed V<sub>max</sub> and consequently, a seemingly lower k<sub>cat</sub>. The mechanism of substrate inhibition can vary, sometimes involving competitive inhibition, uncompetitive inhibition, or mixed inhibition.
2. Enzyme Aggregation:
High substrate concentrations might promote enzyme aggregation, leading to a reduction in the number of active enzyme molecules available for catalysis. Aggregation can alter enzyme conformation, obstructing active sites or preventing substrate binding altogether. This effectively decreases the active enzyme concentration ([E]<sub>T</sub>), leading to a lower observed k<sub>cat</sub>.
3. Product Inhibition:
Although not directly related to substrate concentration, the accumulation of products at high substrate concentrations (due to increased reaction rate) can lead to product inhibition. The product might bind to the enzyme, competing with the substrate or altering the enzyme's conformation. This indirectly affects the observed turnover number by reducing the effective V<sub>max</sub>.
4. Experimental Limitations:
It's essential to acknowledge that measuring V<sub>max</sub> and thus k<sub>cat</sub> accurately can be challenging. At extremely high substrate concentrations, experimental limitations, such as inaccuracies in concentration measurements or difficulties in rapidly mixing reactants, can lead to inaccurate estimations of V<sub>max</sub> and a seemingly altered k<sub>cat</sub>.
5. Allosteric Effects:
Some enzymes exhibit allosteric regulation, where the binding of molecules at a site other than the active site influences the enzyme's activity. High substrate concentrations might lead to allosteric effects that either enhance or inhibit enzyme activity, indirectly influencing the observed k<sub>cat</sub>.
The Importance of Considering K<sub>m</sub>
The Michaelis constant (K<sub>m</sub>) provides critical information about the enzyme's affinity for its substrate. A low K<sub>m</sub> indicates high affinity, implying that the enzyme requires a lower substrate concentration to reach half of its V<sub>max</sub>. Conversely, a high K<sub>m</sub> indicates low affinity, requiring a higher substrate concentration for half-maximal velocity. Understanding K<sub>m</sub> helps to interpret the observed effects of substrate concentration on the turnover number.
Experimental Strategies for Determining k<sub>cat</sub>
Accurate determination of k<sub>cat</sub> requires careful experimental design and execution. Common techniques include:
- Initial rate measurements: Measuring the reaction rate at several substrate concentrations, allowing the construction of a Michaelis-Menten plot to determine V<sub>max</sub>.
- Enzyme assays: Various assays are available to monitor the conversion of substrate to product, ensuring accurate measurement of reaction velocity.
- Spectrophotometry: Useful for monitoring changes in absorbance or fluorescence associated with the reaction, enabling continuous monitoring of reaction progress.
- Data analysis software: Specialized software packages assist in fitting kinetic data to the Michaelis-Menten equation or other appropriate models, ensuring accurate determination of kinetic parameters.
Conclusion: Theoretical vs. Observed k<sub>cat</sub>
In summary, while the theoretical turnover number (k<sub>cat</sub>) is a constant reflecting the intrinsic catalytic efficiency of an enzyme at saturation, the observed k<sub>cat</sub> can be influenced by several factors associated with high substrate concentrations. Substrate inhibition, enzyme aggregation, product inhibition, experimental limitations, and allosteric effects can all contribute to a deviation from the theoretical value. Therefore, it's crucial to carefully consider these factors when interpreting experimental data and evaluating the impact of substrate concentration on enzyme activity. A thorough understanding of enzyme kinetics, particularly the Michaelis-Menten equation and the various factors affecting the observed rate, is vital for accurately assessing the efficiency and behavior of enzymes under different conditions. Analyzing data obtained across a range of substrate concentrations, coupled with rigorous experimental techniques and careful data interpretation, allows for a comprehensive understanding of the enzyme's catalytic properties and its response to varying substrate levels.
Latest Posts
Latest Posts
-
Is Soil Renewable Or Nonrenewable Resource
Mar 26, 2025
-
Are The Initial Velocities On An Uncompetitive Inhibitor The Same
Mar 26, 2025
-
What Is The Relationship Between Cells And Tissues
Mar 26, 2025
-
Where Does Cellular Respiration Take Place In A Eukaryotic Cell
Mar 26, 2025
-
How To Find Eigenvalues And Eigenvectors Of A 4x4 Matrix
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Turnover Number Affected By Substrate Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
