Is Water A Element Compound Or Mixture
Muz Play
Mar 23, 2025 · 7 min read
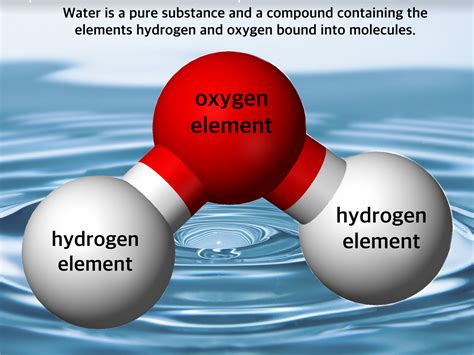
Table of Contents
Is Water an Element, Compound, or Mixture? A Deep Dive into the Nature of H₂O
Water. A substance so ubiquitous, so fundamental to life, that we often take it for granted. But have you ever stopped to consider what water actually is at a fundamental chemical level? Is it an element, a compound, or a mixture? The answer, while seemingly simple, unveils a deeper understanding of chemistry and the building blocks of our world. This comprehensive guide will explore the nature of water, differentiating it from elements and mixtures, and highlighting its unique properties.
Understanding the Basics: Elements, Compounds, and Mixtures
Before diving into the specifics of water, let's clarify the distinctions between elements, compounds, and mixtures. These are fundamental concepts in chemistry that provide the framework for understanding the composition of matter.
Elements: The Fundamental Building Blocks
Elements are pure substances that cannot be broken down into simpler substances by chemical means. They are composed of only one type of atom. The periodic table organizes all known elements, each represented by a unique symbol (e.g., H for hydrogen, O for oxygen). Elements are the fundamental building blocks from which all matter is constructed.
Compounds: Elements United
Compounds are substances formed when two or more different elements chemically combine in fixed proportions. This chemical combination involves the sharing or transfer of electrons, creating strong chemical bonds. Unlike mixtures, the elements in a compound lose their individual properties and form a new substance with unique characteristics. The properties of a compound are distinct from the properties of its constituent elements. For example, sodium (a highly reactive metal) and chlorine (a poisonous gas) combine to form sodium chloride (table salt), a harmless crystalline solid. The chemical formula of a compound represents the ratio of elements present (e.g., H₂O for water, NaCl for sodium chloride).
Mixtures: A Blend of Substances
Mixtures are combinations of two or more substances that are not chemically bonded. The substances in a mixture retain their individual properties, and their proportions can vary. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). The components of a mixture can be separated by physical means, such as filtration, distillation, or evaporation.
Water: A Compound, Not an Element or a Mixture
Now, let's address the central question: Is water an element, compound, or mixture? The answer is unequivocally: water is a compound.
Water's chemical formula, H₂O, clearly indicates that it's composed of two different elements: hydrogen (H) and oxygen (O). These elements are chemically bonded together, forming a molecule with distinct properties unlike those of hydrogen gas or oxygen gas. You cannot simply separate water into hydrogen and oxygen by physical means; you need a chemical reaction (like electrolysis) to break the bonds between the hydrogen and oxygen atoms.
Why water is NOT an element: Water contains two different types of atoms (hydrogen and oxygen), while elements are defined as containing only one type of atom.
Why water is NOT a mixture: The hydrogen and oxygen atoms in water are chemically bonded, forming a unique substance with properties different from its constituent elements. In mixtures, the components retain their individual properties. You cannot separate water into hydrogen and oxygen simply by physical means like filtering or evaporation.
The Unique Properties of Water: A Testament to its Compound Nature
Water's extraordinary properties are a direct consequence of its molecular structure and the strong hydrogen bonds that exist between water molecules. These properties are crucial for life as we know it and are a direct result of its being a compound, not an element or mixture.
High Specific Heat Capacity: A Climate Regulator
Water has an exceptionally high specific heat capacity, meaning it takes a considerable amount of heat energy to raise its temperature. This property is vital for regulating Earth's climate, as large bodies of water absorb and release heat slowly, mitigating temperature fluctuations. This property stems from the strong hydrogen bonds between water molecules, which require significant energy to break.
High Heat of Vaporization: Cooling Effect
Water also possesses a high heat of vaporization, meaning it requires a large amount of energy to change from a liquid to a gas (vaporization). This property is essential for evaporative cooling, a process crucial for maintaining body temperature in many organisms. Again, the strong hydrogen bonds are responsible for this phenomenon.
Universal Solvent: Dissolving Power
Water's exceptional dissolving power, making it a "universal solvent," results from its polar nature. The slightly positive hydrogen atoms and slightly negative oxygen atom in a water molecule create a dipole, attracting and dissolving many ionic and polar substances. This crucial property is central to numerous biological processes, where water acts as a medium for transporting nutrients and waste products.
Density Anomaly: Ice Floats
Water exhibits an unusual density anomaly: ice is less dense than liquid water. This property is crucial for aquatic life, as ice floating on the surface insulates the water below, preventing it from freezing solid and allowing aquatic organisms to survive in winter. This is a result of the unique arrangement of water molecules in the ice crystal lattice.
Cohesion and Adhesion: Surface Tension and Capillary Action
Water molecules are strongly attracted to each other (cohesion) and to other polar substances (adhesion). These properties are responsible for phenomena like surface tension (allowing insects to walk on water) and capillary action (allowing water to move against gravity in plants).
Further Exploring the Chemistry of Water
Understanding the chemical composition of water goes beyond simply stating that it's a compound. It requires delving into the intricacies of its molecular structure and the forces that govern its behaviour.
The Water Molecule: A Polar Compound
The water molecule (H₂O) is a bent molecule, with the oxygen atom at the center and the two hydrogen atoms bonded to it at an angle of approximately 104.5 degrees. This bent shape and the difference in electronegativity between oxygen and hydrogen create a polar molecule, meaning it has a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom).
Hydrogen Bonding: A Powerful Force
The polarity of the water molecule leads to the formation of hydrogen bonds – relatively strong intermolecular forces between the slightly positive hydrogen atom of one water molecule and the slightly negative oxygen atom of another. These hydrogen bonds are responsible for many of water's unique properties, including its high specific heat capacity, high heat of vaporization, and its ability to act as a solvent.
Isotopes of Hydrogen and Oxygen: Variations in Water
While the most common form of water is composed of ¹H and ¹⁶O, variations exist due to the presence of isotopes. Deuterium (²H or D), a heavier isotope of hydrogen, and ¹⁸O, a heavier isotope of oxygen, can be incorporated into water molecules, resulting in different forms of water, such as heavy water (D₂O). These variations have slightly different properties compared to ordinary water.
Conclusion: Water – A Simple Compound with Profound Implications
In conclusion, water is definitively a compound, not an element or a mixture. Its chemical composition, H₂O, represents a chemical union of hydrogen and oxygen atoms, resulting in a substance with unique properties that are essential for life and significantly shape our planet. Understanding water's chemical nature, including its polarity, hydrogen bonding, and unique properties, is crucial for appreciating its fundamental role in chemistry, biology, and environmental science. The seemingly simple molecule of water opens a window to a fascinating world of chemical interactions and their profound influence on our planet and all life within it. The simplicity of its formula belies the remarkable complexity and significance of this ubiquitous and vital substance.
Latest Posts
Latest Posts
-
What Are The 3 Parts Of Atp
Mar 24, 2025
-
Does Covalent Compounds Dissolve In Water
Mar 24, 2025
-
What Color Does Magnesium Metal Burn
Mar 24, 2025
-
How To Find The Basis Of A Subspace
Mar 24, 2025
-
Bone Tissue Can Be Described As
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
