Does Covalent Compounds Dissolve In Water
Muz Play
Mar 24, 2025 · 5 min read
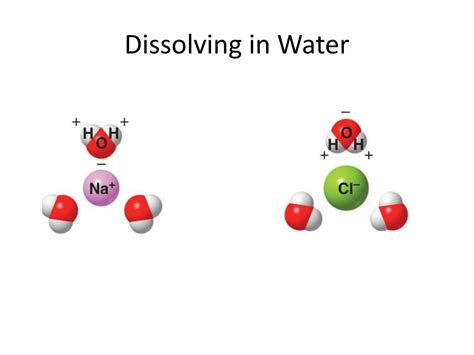
Table of Contents
Do Covalent Compounds Dissolve in Water? A Deep Dive into Solubility
The question of whether covalent compounds dissolve in water is not a simple yes or no. While the general rule of thumb is that ionic compounds dissolve in water and covalent compounds don't, the reality is far more nuanced. The solubility of a covalent compound in water depends on several factors, making it a fascinating area of chemistry. This article will delve deep into the intricacies of covalent compound solubility, exploring the underlying principles and exceptions to the rule.
Understanding the Nature of Covalent Bonds and Water Molecules
Before diving into the solubility of covalent compounds, it's crucial to understand the nature of both covalent bonds and water molecules themselves.
Covalent Bonds: Sharing is Caring
Covalent bonds are formed when two atoms share one or more pairs of electrons. This sharing creates a relatively strong bond, holding the atoms together in a molecule. The strength of this bond varies depending on the atoms involved and the number of shared electron pairs. Unlike ionic bonds, where electrons are transferred completely, covalent bonds result in a more even distribution of electrons, although this distribution can be uneven, leading to polar covalent bonds.
Water: A Polar Wonder
Water (H₂O) is a polar molecule. This means that the distribution of electrons within the molecule is uneven. The oxygen atom is more electronegative than the hydrogen atoms, meaning it attracts the shared electrons more strongly. This creates a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. This polarity is crucial to understanding how water interacts with other molecules.
The Role of Polarity in Solubility: "Like Dissolves Like"
The solubility of a substance in water is largely governed by the principle of "like dissolves like." Polar substances tend to dissolve in polar solvents like water, while nonpolar substances tend to dissolve in nonpolar solvents.
Polar Covalent Compounds and Water
Polar covalent compounds possess a similar uneven distribution of electrons as water. This allows them to interact with water molecules through dipole-dipole interactions and hydrogen bonding. These interactions are relatively strong and can overcome the attractive forces within the covalent compound, allowing it to dissolve. Examples of polar covalent compounds that readily dissolve in water include:
- Sugars (e.g., glucose, sucrose): These contain numerous hydroxyl (-OH) groups, which are highly polar and can form hydrogen bonds with water.
- Alcohols (e.g., ethanol, methanol): The hydroxyl group in alcohols contributes to their polarity and water solubility.
- Carboxylic acids (e.g., acetic acid): The carboxyl group (-COOH) is highly polar and can participate in hydrogen bonding.
- Amines (e.g., methylamine): The amino group (-NH₂) is polar and capable of hydrogen bonding.
Nonpolar Covalent Compounds and Water
Nonpolar covalent compounds have an even distribution of electrons and lack the significant charge separation seen in polar molecules. They cannot form strong interactions with water molecules, resulting in limited solubility. The attractive forces between the nonpolar molecules are stronger than the weak interactions they can form with water. Examples of nonpolar covalent compounds that are largely insoluble in water include:
- Alkanes (e.g., methane, ethane): These consist solely of carbon-carbon and carbon-hydrogen bonds, which are nonpolar.
- Fats and oils: These are composed of long hydrocarbon chains, making them highly nonpolar.
- Many organic solvents (e.g., benzene, toluene): These have predominantly nonpolar bonds.
Factors Affecting Covalent Compound Solubility in Water
While polarity plays a dominant role, several other factors influence the solubility of covalent compounds in water:
1. Molecular Size and Shape:
Larger molecules generally have lower solubility. The increased surface area of larger molecules may lead to more interactions with water molecules, but the increased strength of the van der Waals forces holding the molecule together often outweighs this effect. Similarly, the shape of the molecule can affect how effectively it interacts with water.
2. Hydrogen Bonding:
The presence and number of hydrogen bond donors and acceptors in a molecule significantly affect its water solubility. Molecules with many hydroxyl (-OH), amino (-NH₂), or carboxyl (-COOH) groups can form numerous hydrogen bonds with water, enhancing their solubility.
3. Temperature:
Temperature generally increases the solubility of most solids and liquids in water. Increased kinetic energy at higher temperatures helps overcome the intermolecular forces holding the solute together.
4. Pressure:
Pressure has a minimal effect on the solubility of liquids and solids in water, but significantly impacts the solubility of gases. Increasing the pressure increases the solubility of gases.
Exceptions and Complexities:
The "like dissolves like" rule is a helpful guideline, but not an absolute law. Several factors can lead to exceptions:
- Amphiphilic molecules: These molecules possess both polar and nonpolar regions. They can form micelles or bilayers in water, with the polar regions interacting with water and the nonpolar regions clustering together. Soaps and detergents are prime examples of amphiphilic molecules.
- Intramolecular Hydrogen Bonding: In some cases, strong intramolecular hydrogen bonding can hinder intermolecular hydrogen bonding with water, reducing solubility.
- Steric Hindrance: Bulky substituents can sterically hinder the interaction of polar functional groups with water, decreasing solubility.
Applications and Significance:
Understanding the solubility of covalent compounds in water is crucial across numerous fields:
- Pharmaceutical industry: Solubility is critical for the bioavailability of drugs. Drugs need to dissolve in water to be absorbed into the bloodstream.
- Environmental science: The solubility of pollutants in water determines their fate and transport in the environment.
- Chemical engineering: Solubility is essential in designing and optimizing various chemical processes.
- Food science: Solubility affects the texture, taste, and stability of food products.
Conclusion:
The solubility of covalent compounds in water is a complex phenomenon influenced by numerous factors, primarily the polarity of the molecule. While polar covalent compounds tend to dissolve readily in water due to strong interactions with water molecules, nonpolar compounds generally exhibit low solubility. However, exceptions exist, highlighting the need for a more nuanced understanding of the interplay between molecular structure, intermolecular forces, and the solvent properties of water. Careful consideration of these factors is essential in diverse fields, ranging from pharmaceutical development to environmental monitoring. Further research and investigation continue to refine our understanding of this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
Como Sacar El Diametro De Un Circulo
Mar 26, 2025
-
How To Find All Zeros Of A Polynomial
Mar 26, 2025
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
-
Mendels Dihybrid Crosses Supported The Independent Hypothesis
Mar 26, 2025
-
Is Kinetic Energy Conserved In An Elastic Collision
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Does Covalent Compounds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
