Labeled Periodic Table Metals Nonmetals And Metalloids
Muz Play
Mar 25, 2025 · 7 min read
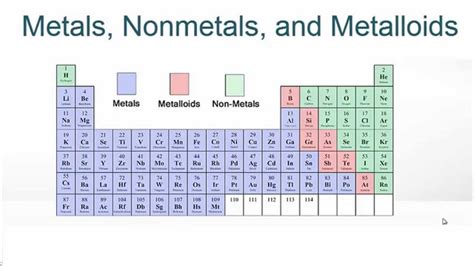
Table of Contents
The Labeled Periodic Table: Metals, Nonmetals, and Metalloids
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One of the most fundamental ways to categorize elements is by their classification as metals, nonmetals, or metalloids. Understanding these classifications is key to comprehending the diverse chemical behavior and applications of elements. This article will delve into the characteristics of each group, explore their placement on the periodic table, and highlight their significance in various fields.
What is a Metal?
Metals constitute the vast majority of elements on the periodic table. They are located to the left of the metalloid staircase (a zig-zag line separating metals from nonmetals). Their defining characteristics stem from their electronic structure, specifically their tendency to lose electrons and form positive ions (cations). This characteristic drives their physical and chemical properties.
Properties of Metals:
- High electrical conductivity: Metals are excellent conductors of electricity due to the presence of delocalized electrons, which can move freely throughout the metallic lattice. This property is crucial in electrical wiring, electronics, and numerous other applications.
- High thermal conductivity: Similar to electrical conductivity, metals efficiently transfer heat. This makes them ideal for applications requiring heat transfer, such as cookware, heat sinks, and engine components.
- Malleability and ductility: Metals can be hammered into thin sheets (malleability) and drawn into wires (ductility) without breaking. This is due to the ability of metal atoms to slide past each other within the metallic structure. This property is extensively utilized in manufacturing processes.
- Luster: Most metals possess a characteristic metallic luster, or shine. This is a result of the interaction of light with the delocalized electrons.
- High tensile strength: Many metals exhibit high tensile strength, meaning they can withstand significant pulling forces before breaking. This strength is vital in construction, engineering, and manufacturing.
- High density: Generally, metals possess higher densities compared to nonmetals. This is due to the close packing of atoms in the metallic lattice.
- Sonorous: When struck, most metals produce a ringing sound (sonorous).
Examples of Metals and Their Uses:
- Iron (Fe): A crucial component in steel, used in construction, automobiles, and countless other applications. Its strength and relative abundance make it incredibly important.
- Aluminum (Al): Lightweight, corrosion-resistant, and highly conductive, aluminum is widely used in aircraft, packaging, and electrical wiring.
- Copper (Cu): An excellent conductor of electricity and heat, copper is essential in electrical wiring, plumbing, and electronics.
- Gold (Au): Highly valued for its inertness, malleability, and luster, gold is used in jewelry, electronics, and investments.
- Silver (Ag): Another excellent conductor, silver finds applications in electronics, photography, and silverware.
- Sodium (Na): While highly reactive, sodium plays crucial roles in sodium-vapor lamps and certain chemical processes.
What is a Nonmetal?
Nonmetals are situated on the right side of the periodic table, primarily above the metalloid staircase. Unlike metals, they generally lack the characteristic metallic properties. Their chemical behavior is dominated by their tendency to gain electrons and form negative ions (anions).
Properties of Nonmetals:
- Poor electrical conductivity: Nonmetals are generally poor conductors of electricity because they lack freely moving electrons. Exceptions exist, such as graphite (a form of carbon).
- Poor thermal conductivity: Similarly, nonmetals are poor conductors of heat.
- Brittle: Nonmetals are often brittle and tend to shatter when subjected to stress.
- Dull appearance: They lack the metallic luster characteristic of metals.
- Low density: Nonmetals usually have lower densities than metals.
- Low melting and boiling points: Compared to metals, nonmetals typically have lower melting and boiling points.
- Gain electrons easily: This tendency to gain electrons is a key defining characteristic, leading to the formation of covalent bonds.
Examples of Nonmetals and Their Uses:
- Oxygen (O): Essential for respiration and combustion, oxygen is vital for life and numerous industrial processes.
- Carbon (C): A fundamental element in organic chemistry, carbon forms the basis of life and is crucial in materials like diamonds, graphite, and plastics.
- Nitrogen (N): A major component of the atmosphere, nitrogen is crucial in fertilizers and the production of ammonia.
- Chlorine (Cl): Used in water purification and as a component in various chemicals, chlorine is a powerful disinfectant.
- Sulfur (S): Used in the production of sulfuric acid, a crucial industrial chemical, sulfur also has applications in fertilizers and vulcanization of rubber.
- Fluorine (F): Used in dental products and various industrial applications, fluorine is a highly reactive element.
What is a Metalloid?
Metalloids, also known as semimetals, occupy a fascinating intermediate position on the periodic table, residing along the metalloid staircase. They exhibit properties that are intermediate between those of metals and nonmetals, making them unique and valuable.
Properties of Metalloids:
- Semiconductors: The most significant property of metalloids is their semiconducting behavior. Their electrical conductivity lies between that of metals and nonmetals; it increases with increasing temperature. This property is crucial in the electronics industry.
- Variable properties: Their properties can vary significantly depending on their crystalline structure and purity, leading to diverse applications.
- Brittle: Similar to nonmetals, metalloids are generally brittle.
Examples of Metalloids and Their Uses:
- Silicon (Si): The most abundant metalloid, silicon is the foundation of the semiconductor industry, used in transistors, integrated circuits, and solar cells.
- Germanium (Ge): Used in transistors and other semiconductor devices, germanium was historically important but has been largely replaced by silicon in many applications.
- Arsenic (As): Used in some semiconductors and also as a doping agent in silicon-based electronics. It's also used in certain alloys.
- Antimony (Sb): Used in alloys, particularly in lead-based alloys for batteries and solder. It also has applications in flame retardants.
- Tellurium (Te): Used in solar cells, some alloys, and as a component in certain semiconductors.
- Boron (B): Used in some semiconductors, especially in high-temperature applications. It also has uses in other materials science applications.
The Periodic Table and Element Classification: A Visual Guide
The periodic table visually represents the organization of elements based on their atomic number and electron configuration. The horizontal rows are called periods, and the vertical columns are called groups or families. Elements within the same group share similar chemical properties due to their similar outer electron configurations. The metalloid staircase serves as a visual boundary separating metals, nonmetals, and metalloids. While this staircase provides a general guideline, the classification of some elements near the boundary can be somewhat ambiguous.
The Importance of Understanding Metal, Nonmetal, and Metalloid Classification
The classification of elements as metals, nonmetals, or metalloids is not simply an academic exercise. This categorization provides a fundamental framework for understanding the chemical behavior and physical properties of elements, which are essential in:
- Materials science: The selection of materials for specific applications relies heavily on understanding the properties of metals, nonmetals, and metalloids. For example, the choice of a metal for a structural component depends on its strength, density, and corrosion resistance. The choice of a semiconductor for electronics depends on its band gap and conductivity.
- Chemical engineering: Understanding the reactivity of different elements is crucial in chemical reactions and industrial processes. The use of metals in catalysis or the use of nonmetals in chemical synthesis requires a deep understanding of their chemical behavior.
- Medicine: Many elements and compounds based on metals and nonmetals play crucial roles in medical applications, from diagnostic imaging to drug delivery.
- Electronics: The semiconductor industry is entirely reliant on the properties of metalloids like silicon and germanium.
- Environmental science: Understanding the environmental impact of metals, nonmetals, and metalloids is vital for pollution control and resource management.
Conclusion
The periodic table's classification of elements as metals, nonmetals, and metalloids provides a fundamental framework for understanding the vast diversity of chemical behavior and physical properties exhibited by elements. Each classification encompasses a broad range of elements with unique characteristics and applications, contributing significantly to various scientific and technological advancements. A thorough understanding of these classifications is crucial for any serious pursuit of chemistry or related fields. From the construction of skyscrapers to the development of advanced electronics, the properties of metals, nonmetals, and metalloids are essential to modern society. Further exploration into the individual elements within these categories will reveal an even deeper appreciation for the intricacies of the periodic table and the fundamental building blocks of matter.
Latest Posts
Latest Posts
-
Solving Linear Systems With Graphing 7 1
Mar 27, 2025
-
Compare And Contrast Sexual Reproduction And Asexual Reproduction
Mar 27, 2025
-
Interval Of Convergence For Taylor Series
Mar 27, 2025
-
The Martian And The Car Answer Key
Mar 27, 2025
-
Rusting Of Iron Chemical Or Physical Change
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Labeled Periodic Table Metals Nonmetals And Metalloids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
