Lewis Structure Of Co With Formal Charges
Muz Play
Mar 23, 2025 · 6 min read
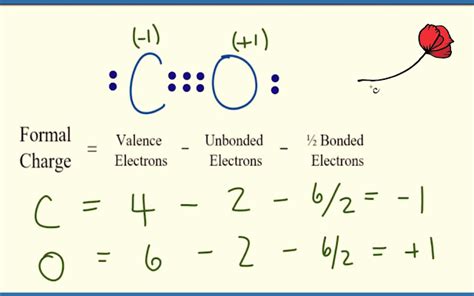
Table of Contents
Lewis Structure of CO with Formal Charges: A Deep Dive
The seemingly simple molecule of carbon monoxide (CO) presents a fascinating case study in understanding Lewis structures and formal charges. Its unusual bonding and the need to account for formal charges to accurately represent its electronic structure provide valuable insights into chemical bonding principles. This article will delve deep into constructing the Lewis structure of CO, explaining the reasoning behind each step, and comprehensively exploring the concept of formal charges within the context of this molecule. We'll also touch on the implications of the formal charges for the molecule's properties.
Understanding Lewis Structures
Before diving into the intricacies of CO's Lewis structure, let's briefly revisit the fundamental principles of Lewis structures. These diagrams provide a simplified representation of a molecule's valence electrons and bonding. They help us visualize the distribution of electrons, predicting molecular geometry and properties. Key elements involved in drawing Lewis structures include:
- Valence Electrons: The number of electrons in the outermost shell of an atom, which participate in bonding.
- Octet Rule: Atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons (except for hydrogen and helium, which follow the duet rule).
- Lone Pairs: Pairs of valence electrons not involved in bonding.
- Bonding Pairs: Pairs of valence electrons shared between two atoms, representing a single bond.
Constructing the Lewis Structure of CO
-
Counting Valence Electrons: Carbon has four valence electrons, and oxygen has six. Therefore, the CO molecule has a total of 10 valence electrons (4 + 6 = 10).
-
Identifying the Central Atom: In this case, carbon is slightly less electronegative than oxygen, making it the central atom. However, both atoms participate significantly in the bonding.
-
Initial Skeleton Structure: We begin with a single bond between the carbon and oxygen atoms: C-O. This utilizes two of the 10 valence electrons.
-
Distributing Remaining Electrons: We distribute the remaining eight electrons (10 - 2 = 8) as lone pairs around the atoms to satisfy the octet rule (or duet rule for hydrogen if present). Placing three lone pairs on oxygen and one lone pair on carbon gives:
:C≡O: -
Checking the Octet Rule: Oxygen now has eight electrons (two in the bond and six in lone pairs), satisfying the octet rule. However, carbon only has four electrons. To satisfy the octet rule for carbon, we need to form a triple bond:
:C≡O:
Now both carbon and oxygen have a full octet.
Formal Charges: Calculating and Interpreting
Formal charges are a tool to assess the distribution of electrons in a Lewis structure. They help determine the most plausible structure when multiple structures are possible. The formal charge of an atom is calculated using the following formula:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (½ Bonding Electrons)
Let's calculate the formal charges for both carbon and oxygen in the CO triple bond structure:
Carbon:
- Valence Electrons = 4
- Non-bonding Electrons = 2 (one lone pair)
- Bonding Electrons = 6 (triple bond)
- Formal Charge = 4 - 2 - (6/2) = 0
Oxygen:
- Valence Electrons = 6
- Non-bonding Electrons = 4 (two lone pairs)
- Bonding Electrons = 6 (triple bond)
- Formal Charge = 6 - 4 - (6/2) = 0
The formal charges for both carbon and oxygen are zero in the triple bond structure. This indicates a relatively stable and likely structure for carbon monoxide.
Alternative Lewis Structures and Their Formal Charges
While the triple bond structure is the most stable and accurate representation of CO, it's crucial to consider alternative structures to understand the concept of formal charges fully. Let's explore a few possibilities:
Structure 1: Single Bond
:C-O:
- Carbon Formal Charge: 4 - 2 - (2/2) = +2
- Oxygen Formal Charge: 6 - 6 - (2/2) = -2
This structure has significant formal charges, making it significantly less stable than the triple bond structure.
Structure 2: Double Bond
:C=O:
- Carbon Formal Charge: 4 - 2 - (4/2) = +1
- Oxygen Formal Charge: 6 - 4 - (4/2) = -1
This structure also possesses formal charges, although smaller than in Structure 1, making it less stable than the triple bond structure.
Importance of Formal Charges in Choosing the Best Lewis Structure
Comparing the three structures, it is clear that the triple bond structure with zero formal charges on both atoms is the most likely and stable arrangement. Formal charges help us to:
- Identify the most plausible structure: Structures with smaller formal charges are generally more stable.
- Predict reactivity: Atoms with significant formal charges may be more reactive.
- Understand bond polarity: Formal charges can indicate the direction of bond polarity.
In the case of CO, the zero formal charges on both carbon and oxygen in the triple bond structure highlight the strong and relatively nonpolar nature of the bond.
Resonance Structures and CO
While the triple bond structure best represents CO, we can also consider resonance structures, which depict the delocalization of electrons. In CO, resonance is less significant than in molecules with multiple equivalent bonding arrangements. However, recognizing the possibility of resonance helps build a comprehensive understanding of the bonding in CO.
Beyond Lewis Structures: Molecular Orbital Theory
While Lewis structures provide a useful simplified model, they don't completely capture the intricacies of bonding in CO. Molecular orbital theory (MOT) offers a more sophisticated description of the bonding, explaining the strength and stability of the triple bond. MOT explains the presence of both sigma and pi bonds in the molecule, offering a more detailed understanding of electron distribution and molecular properties.
Properties of CO Related to its Lewis Structure
The triple bond in CO gives rise to several key properties:
- High Bond Strength: The triple bond is very strong, requiring significant energy to break.
- Short Bond Length: The triple bond pulls the carbon and oxygen atoms closer together than a single or double bond would.
- Low Polarity: Despite the difference in electronegativity, the symmetrical distribution of electrons in the triple bond results in a relatively nonpolar molecule.
- Toxicity: The strong triple bond and ability of CO to bind strongly to hemoglobin contribute to its toxicity by displacing oxygen.
Conclusion
The Lewis structure of CO with its formal charges is a valuable example illustrating the principles of chemical bonding and the importance of using formal charges to choose the most plausible Lewis structure. The triple bond structure with zero formal charges represents the stable structure for CO, reflecting its properties such as high bond strength, short bond length, and relatively nonpolar character. While Lewis structures provide a valuable starting point, understanding more advanced theories like MOT is crucial for a comprehensive grasp of the nuances of chemical bonding in molecules like carbon monoxide. This in-depth analysis of CO not only provides a deeper understanding of its structure but also illustrates the critical role of formal charges in determining molecular stability and properties. Understanding these concepts forms the foundation for further explorations into more complex molecular structures and reactions.
Latest Posts
Latest Posts
-
The Light Dependent Reactions Occur In The Stroma Of The Chloroplast
Mar 25, 2025
-
Name Two Ecological Roles Of Fungi
Mar 25, 2025
-
What Affects The Rate Of Diffusion
Mar 25, 2025
-
Law Of Independent Assortment Vs Law Of Segregation
Mar 25, 2025
-
Amphibians Are Thought To Have Evolved From
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure Of Co With Formal Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
