Lewis Structure Practice Worksheet With Answers
Muz Play
Mar 31, 2025 · 7 min read
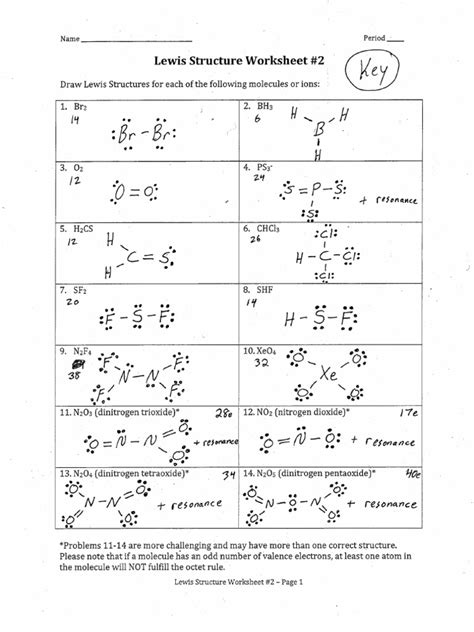
Table of Contents
Lewis Structure Practice Worksheet with Answers: A Comprehensive Guide
Mastering Lewis structures is fundamental to understanding chemistry. They provide a visual representation of the valence electrons in a molecule, crucial for predicting molecular geometry, polarity, and reactivity. This comprehensive guide serves as a Lewis structure practice worksheet with answers, providing numerous examples and explanations to solidify your understanding. We'll cover key concepts, provide step-by-step solutions, and offer tips for tackling even the most challenging structures.
Understanding Lewis Structures: The Basics
Before diving into the practice problems, let's review the core principles behind drawing Lewis structures. A Lewis structure, also known as an electron dot structure, depicts the arrangement of valence electrons (outer shell electrons) around atoms in a molecule. These electrons are represented as dots, with each dot representing a single electron. Pairs of dots represent shared electron pairs (covalent bonds).
Key Steps in Drawing Lewis Structures:
-
Determine the total number of valence electrons: Add up the valence electrons of all atoms in the molecule. Remember to account for the charge if the molecule is an ion.
-
Identify the central atom: Usually, the least electronegative atom (excluding hydrogen) serves as the central atom.
-
Connect atoms with single bonds: Draw single bonds (one electron pair) between the central atom and surrounding atoms.
-
Distribute remaining electrons: Place the remaining valence electrons around the atoms, starting with the outer atoms, to satisfy the octet rule (eight electrons around each atom, except for hydrogen, which follows the duet rule – two electrons).
-
Check for octet rule satisfaction: If any atoms lack an octet, form multiple bonds (double or triple bonds) by moving lone pairs from outer atoms to form additional bonds with the central atom.
-
Formal charge calculation (optional but recommended): Calculate the formal charge of each atom to ensure the most stable structure. The formal charge is calculated as: Formal Charge = Valence Electrons - (Non-bonding Electrons + ½ Bonding Electrons). A stable structure usually minimizes formal charges.
Lewis Structure Practice Problems: Step-by-Step Solutions
Let's work through some examples, illustrating the process and highlighting common pitfalls.
Problem 1: Draw the Lewis structure for water (H₂O).
-
Valence electrons: Oxygen (Group 16) has 6 valence electrons, and each hydrogen (Group 1) has 1. Total: 6 + 1 + 1 = 8 valence electrons.
-
Central atom: Oxygen is the central atom.
-
Single bonds: Connect oxygen to each hydrogen with a single bond (2 electrons used).
-
Remaining electrons: 8 - 2 = 6 electrons remain. Place these as lone pairs around the oxygen atom.
-
Octet rule: Oxygen has 8 electrons (2 in bonds and 6 as lone pairs), and each hydrogen has 2 electrons (a duet). The octet rule is satisfied.
Answer: The Lewis structure for water is:
H
|
H - O - H
Problem 2: Draw the Lewis structure for carbon dioxide (CO₂).
-
Valence electrons: Carbon (Group 14) has 4, and each oxygen (Group 16) has 6. Total: 4 + 6 + 6 = 16 valence electrons.
-
Central atom: Carbon is the central atom.
-
Single bonds: Connect carbon to each oxygen with a single bond (4 electrons used).
-
Remaining electrons: 16 - 4 = 12 electrons. Place these as lone pairs around the oxygen atoms.
-
Octet rule: Oxygen atoms have 8 electrons each, but carbon only has 4. To satisfy the octet rule, we need to form double bonds between carbon and each oxygen. Move two lone pairs from each oxygen to form two double bonds with carbon.
-
Final structure:
Answer: The Lewis structure for carbon dioxide is:
O = C = O
Problem 3: Draw the Lewis structure for ammonia (NH₃).
-
Valence electrons: Nitrogen (Group 15) has 5, and each hydrogen (Group 1) has 1. Total: 5 + 3(1) = 8 valence electrons.
-
Central atom: Nitrogen is the central atom.
-
Single bonds: Connect nitrogen to each hydrogen with a single bond (6 electrons used).
-
Remaining electrons: 8 - 6 = 2 electrons. Place these as a lone pair on the nitrogen atom.
-
Octet rule: Nitrogen has 8 electrons (3 bonds and 1 lone pair), and each hydrogen has 2 electrons. The octet rule is satisfied.
Answer: The Lewis structure for ammonia is:
H
/ |
H-N-H
Problem 4: Draw the Lewis structure for the nitrate ion (NO₃⁻).
-
Valence electrons: Nitrogen (Group 15) has 5, each oxygen (Group 16) has 6, and there's an extra electron due to the negative charge. Total: 5 + 3(6) + 1 = 24 valence electrons.
-
Central atom: Nitrogen is the central atom.
-
Single bonds: Connect nitrogen to each oxygen with a single bond (6 electrons used).
-
Remaining electrons: 24 - 6 = 18 electrons. Place these as lone pairs around the oxygen atoms.
-
Octet rule: Oxygen atoms have 8 electrons, but nitrogen only has 6. To satisfy the octet rule, form a double bond between nitrogen and one of the oxygen atoms. This results in resonance structures.
Answer: The nitrate ion has three resonance structures:
O-
||
O-N-O <-> O=N-O <-> O-N=O
|
O-
O-
O-
Problem 5: Draw the Lewis structure for sulfate ion (SO₄²⁻).
-
Valence electrons: Sulfur (Group 16) has 6, each oxygen (Group 16) has 6, and there are two extra electrons due to the 2- charge. Total: 6 + 4(6) + 2 = 32 valence electrons.
-
Central atom: Sulfur is the central atom.
-
Single bonds: Connect sulfur to each oxygen with a single bond (8 electrons used).
-
Remaining electrons: 32 - 8 = 24 electrons. Place these as lone pairs around the oxygen atoms.
-
Octet rule: Oxygen atoms have 8 electrons each, but sulfur only has 8. To minimize formal charges, form double bonds between sulfur and two of the oxygen atoms, resulting in resonance structures.
Answer: The sulfate ion has multiple resonance structures, with two double bonds and two single bonds, distributing the double bonds around the oxygens.
Advanced Lewis Structures and Exceptions to the Octet Rule
Some molecules don't perfectly adhere to the octet rule. These exceptions include:
-
Electron-deficient molecules: These molecules have fewer than eight electrons around the central atom, often seen in boron compounds (e.g., BF₃).
-
Odd-electron molecules (free radicals): These molecules have an odd number of valence electrons, resulting in an unpaired electron (e.g., NO₂).
-
Expanded octet molecules: These molecules have more than eight electrons around the central atom, typically seen in elements from the third period and beyond (e.g., SF₆).
Problem 6: Draw the Lewis structure for boron trifluoride (BF₃).
Boron has only 3 valence electrons, making it electron-deficient. It forms three single bonds with fluorine, resulting in only 6 electrons around boron.
Answer:
F
/ | \
F-B-F
Problem 7: Draw the Lewis structure for sulfur hexafluoride (SF₆).
Sulfur expands its octet to accommodate 12 electrons around it, forming six single bonds with fluorine.
Answer: (Note: a 3D representation would be more accurate, showing the octahedral geometry)
F
/|\
/ | \
F-S-F
\ | /
\|/
F
Tips for Success with Lewis Structures
-
Practice regularly: The more you practice, the better you'll become at quickly and accurately drawing Lewis structures.
-
Use a systematic approach: Follow the steps outlined above consistently.
-
Check your work: Always verify that the total number of valence electrons is accounted for and that the octet rule (or its exceptions) is satisfied.
-
Consider formal charges: Formal charges help determine the most stable Lewis structure.
-
Utilize online resources: Numerous online tools and tutorials can assist you in learning and practicing.
-
Focus on understanding, not memorization: Focus on grasping the underlying principles, not just rote memorization of structures.
This comprehensive guide, serving as a Lewis structure practice worksheet with answers, provides a solid foundation for understanding and mastering this crucial concept in chemistry. By practicing the examples provided and applying the strategies discussed, you’ll build confidence and proficiency in drawing Lewis structures for a wide variety of molecules and ions. Remember to consult additional resources and seek help when needed. Consistent practice is key to success!
Latest Posts
Latest Posts
-
Why Are Saturated Fats Solid At Room Temperature
Apr 02, 2025
-
How To Divide Exponents In Fractions
Apr 02, 2025
-
For Most Substances Solubility Is Blank As Temperature
Apr 02, 2025
-
Differentiate Between Systemic And Pulmonary Circulation
Apr 02, 2025
-
Graph Of Atomic Number Vs Atomic Radius
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure Practice Worksheet With Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
