List Of Strong Weak Acids And Bases
Muz Play
Mar 15, 2025 · 5 min read
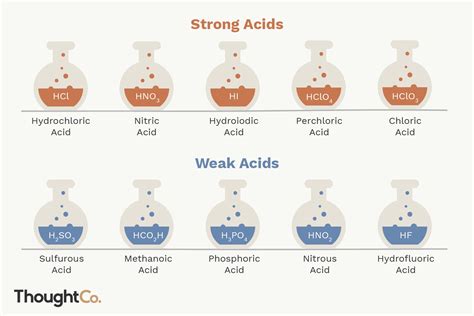
Table of Contents
A Comprehensive List of Strong and Weak Acids and Bases
Understanding the strength of acids and bases is fundamental in chemistry. This knowledge is crucial for predicting reaction outcomes, calculating pH values, and comprehending numerous chemical processes. This article provides a comprehensive list of strong and weak acids and bases, explaining the factors that determine their strength and offering practical examples of their applications.
What Defines a Strong Acid or Base?
The strength of an acid or base is determined by its degree of dissociation or ionization in water. Strong acids and bases completely dissociate into their constituent ions when dissolved in water, while weak acids and bases only partially dissociate. This difference significantly impacts their properties and reactivity.
-
Strong Acids: Completely ionize in water, releasing a high concentration of H⁺ ions (protons). This leads to a low pH (highly acidic).
-
Weak Acids: Partially ionize in water, releasing a low concentration of H⁺ ions. This results in a higher pH compared to strong acids (less acidic).
-
Strong Bases: Completely dissociate in water, releasing a high concentration of OH⁻ ions (hydroxide ions). This leads to a high pH (highly alkaline).
-
Weak Bases: Partially dissociate in water, releasing a low concentration of OH⁻ ions. This results in a lower pH compared to strong bases (less alkaline).
Factors Affecting Acid and Base Strength
Several factors influence the strength of an acid or base:
-
Bond Strength: Weaker bonds between the proton (H⁺) and the rest of the acid molecule lead to easier dissociation, resulting in stronger acids.
-
Electronegativity: Higher electronegativity of the atom bonded to the proton stabilizes the resulting anion, making it easier for the proton to dissociate, hence stronger acidity.
-
Resonance Stabilization: If the conjugate base (the anion formed after proton donation) is resonance-stabilized, the acid will be stronger because the negative charge is delocalized, making it more stable.
-
Inductive Effect: Electron-withdrawing groups increase the acidity by stabilizing the negative charge on the conjugate base.
-
Solvent Effects: The solvent in which the acid or base is dissolved plays a role in its dissociation and strength.
List of Common Strong Acids
Strong acids are relatively few in number. The most common ones include:
- Hydrochloric acid (HCl): Found in gastric acid and used in various industrial processes.
- Hydrobromic acid (HBr): Used in the synthesis of organic compounds.
- Hydroiodic acid (HI): A strong reducing agent.
- Sulfuric acid (H₂SO₄): A highly corrosive acid with extensive industrial applications. Note that only the first proton (H⁺) dissociates completely. The second dissociation is weaker.
- Nitric acid (HNO₃): Used in the production of fertilizers and explosives.
- Perchloric acid (HClO₄): One of the strongest known acids.
List of Common Weak Acids
Weak acids are far more numerous than strong acids. Here's a selection:
- Acetic acid (CH₃COOH): Found in vinegar.
- Formic acid (HCOOH): Found in ant stings.
- Benzoic acid (C₆H₅COOH): A common preservative.
- Citric acid (C₆H₈O₇): Found in citrus fruits.
- Lactic acid (C₃H₆O₃): Produced in muscles during strenuous activity.
- Carbonic acid (H₂CO₃): Found in carbonated drinks and plays a crucial role in blood pH regulation.
- Phosphoric acid (H₃PO₄): Used in fertilizers and food additives. It's a polyprotic acid, meaning it can donate multiple protons, but its strength decreases with each proton donation.
- Hydrofluoric acid (HF): A relatively weak acid despite the high electronegativity of fluorine. The strong H-F bond hinders dissociation.
- Hydrocyanic acid (HCN): A highly toxic weak acid.
List of Common Strong Bases
Strong bases are mostly alkali metal hydroxides and some alkaline earth metal hydroxides.
- Sodium hydroxide (NaOH): Also known as caustic soda, used in soap making and drain cleaners.
- Potassium hydroxide (KOH): Used in the production of soaps and fertilizers.
- Lithium hydroxide (LiOH): Used in batteries and as a desiccant.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime, used in construction and wastewater treatment.
- Barium hydroxide (Ba(OH)₂): Used in analytical chemistry.
List of Common Weak Bases
Weak bases often involve amines and some metal oxides.
- Ammonia (NH₃): A common household cleaner.
- Methylamine (CH₃NH₂): Used in the production of pesticides and pharmaceuticals.
- Ethylamine (C₂H₅NH₂): Used as a solvent and in the production of various chemicals.
- Pyridine (C₅H₅N): A heterocyclic aromatic compound used as a solvent and reagent.
- Aniline (C₆H₅NH₂): Used in the production of dyes and pharmaceuticals.
- Magnesium oxide (MgO): A weakly basic oxide used in various industrial applications.
- Calcium oxide (CaO): A weakly basic oxide used in construction materials.
Applications of Strong and Weak Acids and Bases
The diverse applications of acids and bases highlight their importance in various fields:
-
Industrial Processes: Strong acids like sulfuric acid are extensively used in the production of fertilizers, detergents, and various chemicals. Strong bases like sodium hydroxide are crucial in the paper industry, soap making, and water treatment.
-
Food and Beverages: Weak acids like acetic acid (vinegar), citric acid (citrus fruits), and lactic acid (dairy products) contribute to the flavor and preservation of many food items.
-
Medicine: Acids and bases play vital roles in drug formulation, digestion (hydrochloric acid in the stomach), and regulating blood pH.
-
Analytical Chemistry: Acids and bases are fundamental in titrations, pH measurements, and various analytical techniques.
-
Environmental Science: Understanding acid-base chemistry is crucial for managing water quality, soil acidity, and air pollution.
Safety Precautions When Handling Acids and Bases
Acids and bases can be hazardous. Always follow safety precautions:
- Wear appropriate personal protective equipment (PPE): This includes gloves, eye protection, and lab coats.
- Work in a well-ventilated area: Many acids and bases produce harmful fumes.
- Handle carefully: Avoid spills and direct contact with skin and eyes.
- Neutralize spills properly: Follow established protocols for neutralizing acid or base spills.
- Store safely: Acids and bases should be stored separately and in appropriate containers.
Conclusion
This comprehensive list and discussion of strong and weak acids and bases provide a foundational understanding of their properties, behavior, and applications. Understanding the differences between strong and weak acids and bases is critical for predicting chemical reactions, interpreting experimental results, and ensuring safe handling procedures in various contexts, from industrial settings to everyday life. Remember to always prioritize safety when working with these substances. Further exploration of specific acids and bases can be undertaken using reliable chemistry textbooks and databases.
Latest Posts
Latest Posts
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 15, 2025
-
How Many Unpaired Electrons Are In Sulfur Atom
Mar 15, 2025
-
Similarities Between The Romanticism And Transcendentalism Movement
Mar 15, 2025
-
Made Up Of Two Glucose Polysaccharides Amylose And Amylopectin
Mar 15, 2025
-
What Moves The Fastest In Tlc
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about List Of Strong Weak Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
