Matter Is Not Created Nor Destroyed
Muz Play
Mar 31, 2025 · 6 min read
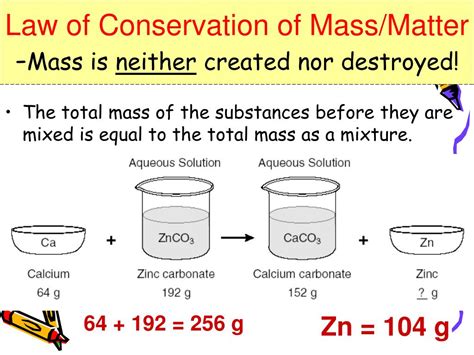
Table of Contents
Matter is Neither Created Nor Destroyed: A Deep Dive into the Law of Conservation of Mass
The principle that "matter is neither created nor destroyed" is a cornerstone of modern science, a fundamental law that governs the universe at its most basic level. This principle, more formally known as the law of conservation of mass, states that the total mass in a closed system remains constant over time, regardless of any transformations or processes that occur within that system. While seemingly simple, this law has profound implications across various scientific disciplines, from chemistry and physics to cosmology and environmental science. This article delves into the nuances of this law, exploring its history, its applications, and some of its apparent exceptions.
A Brief History: From Alchemy to Atomic Theory
The concept of conservation of mass wasn't always a given. Early alchemists, for example, believed that transmutation—the transformation of one substance into another—could create new matter. However, careful experimentation gradually shifted this perspective.
Antoine Lavoisier, often considered the "father of modern chemistry," played a pivotal role in establishing the law of conservation of mass. Through meticulous experiments in the late 18th century, particularly involving meticulously weighing reactants and products in chemical reactions, he demonstrated that mass remains constant throughout chemical processes. Lavoisier's work, based on precise quantitative measurements, was crucial in establishing the law as a fundamental scientific principle. His famous quote, "Nothing is lost, nothing is created, everything is transformed," elegantly summarizes the essence of the law.
This principle was further solidified with the development of atomic theory. John Dalton's atomic theory, proposed in the early 19th century, postulated that matter is composed of indivisible atoms that combine in simple whole-number ratios to form compounds. This neatly aligned with the law of conservation of mass, providing a microscopic explanation for the macroscopic observation of constant mass in chemical reactions. Atoms, according to Dalton, were neither created nor destroyed in chemical reactions; they were simply rearranged.
The Law in Action: Examples Across Scientific Fields
The law of conservation of mass manifests itself in a myriad of everyday phenomena and scientific processes.
Chemistry: Chemical Reactions and Equations
In chemistry, the law is fundamental to balancing chemical equations. Every chemical reaction involves a rearrangement of atoms, but the total number of each type of atom remains the same on both sides of the equation. For example, in the reaction between hydrogen and oxygen to form water (2H₂ + O₂ → 2H₂O), the total mass of hydrogen and oxygen before the reaction equals the total mass of water produced. This is a direct manifestation of the law of conservation of mass.
Physics: Nuclear Reactions and Mass-Energy Equivalence
While the law of conservation of mass holds true for most chemical reactions, things become slightly more complex when considering nuclear reactions. In nuclear reactions, a small amount of mass can be converted into energy (or vice versa), according to Einstein's famous equation, E=mc². This equation demonstrates the equivalence of mass and energy, suggesting that mass can be converted into energy and vice versa. This means that the total mass in a nuclear reaction might not appear strictly constant, but the total mass-energy remains conserved.
Nuclear fission, for instance, involves the splitting of a heavy atomic nucleus, resulting in the release of a significant amount of energy. A small portion of the original mass is converted into this energy. Similarly, nuclear fusion, the process that powers the sun, involves the fusion of lighter nuclei into a heavier nucleus, also releasing a tremendous amount of energy and exhibiting a small mass deficit. Even in these cases, though, the total mass-energy of the system is conserved.
Environmental Science: Pollution and Waste Management
The law of conservation of mass is crucial in understanding environmental issues. Pollutants, for example, are not destroyed; they are merely transformed or transferred from one form or location to another. Waste management strategies rely heavily on this principle: waste must be treated and disposed of responsibly, ensuring its safe containment and preventing its harmful dispersion into the environment. This includes careful consideration of the overall mass balance to prevent environmental contamination.
Cosmology: The Formation and Evolution of the Universe
The early universe, according to the Big Bang theory, began as a singularity of incredibly high density and temperature. Even though the universe has expanded immensely since its inception, the total mass-energy remains constant. The energy associated with the expansion of the universe is balanced against the total mass of matter and dark matter within the universe. While the distribution of mass and energy changed dramatically throughout the universe's evolution, the overall amount remained conserved.
Apparent Exceptions and Nuances
While the law of conservation of mass is a robust principle, some situations might appear to contradict it. However, closer examination often reveals that the law remains valid if all aspects of the system are considered.
Open Systems versus Closed Systems
The law of conservation of mass applies strictly to closed systems, systems that do not exchange matter with their surroundings. In an open system, where matter can enter or leave, the total mass within the system may change. For instance, a pot of boiling water on a stove is an open system; as the water evaporates, the mass of the system decreases. However, if the water vapor is accounted for, the total mass of the system (water + water vapor) remains constant.
Extremely High Energies and Relativistic Effects
At extremely high energies, as encountered in particle physics experiments, relativistic effects become significant. Here, the conversion of mass to energy (and vice versa) becomes more pronounced, leading to apparent variations in mass. However, even in such cases, the total mass-energy remains conserved, upholding the broader principle of conservation.
The Broader Significance of Conservation Laws
The law of conservation of mass is not an isolated principle; it's one of several fundamental conservation laws in physics, including conservation of energy, momentum, and charge. These laws represent powerful symmetries of the universe, reflecting underlying invariances and suggesting that the fundamental laws of physics remain the same regardless of time or location. The existence of such fundamental conservation laws significantly simplifies our understanding of the universe and provides a framework for predicting and explaining numerous physical phenomena.
The law's importance extends beyond the purely scientific realm. Understanding the principle of conservation of mass is crucial in various technological applications, such as industrial processes, environmental monitoring, and waste management. It provides a crucial foundation for making informed decisions regarding resource utilization and environmental protection.
Conclusion: A Timeless Principle
The law of conservation of mass, a cornerstone of scientific understanding, asserts that matter is neither created nor destroyed, only transformed. While refinements are needed to account for relativistic effects in high-energy scenarios, the underlying principle remains remarkably robust and accurate in a vast majority of physical and chemical processes. Its influence is far-reaching, impacting our comprehension of everything from chemical reactions to the evolution of the universe itself, making it a testament to the elegance and consistency of the laws governing our reality. The law’s continued relevance reinforces its position as a fundamental pillar of modern science and a cornerstone for understanding the world around us. Further research and technological advancements will undoubtedly continue to refine our understanding of this profound principle, reaffirming its central role in scientific thought and technological progress.
Latest Posts
Latest Posts
-
How Does The Nucleus And Ribosomes Work Together
Apr 01, 2025
-
Having A Single Set Of Unpaired Chromosomes
Apr 01, 2025
-
How To Calculate Current In A Series Circuit
Apr 01, 2025
-
Unity Of Life And Diversity Of Life
Apr 01, 2025
-
Choose The Most Likely Correlation Value For This Scatterplot
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Matter Is Not Created Nor Destroyed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
