Mixing An Acid And A Base
Muz Play
Mar 28, 2025 · 5 min read
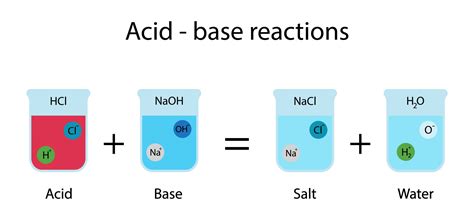
Table of Contents
Mixing an Acid and a Base: A Deep Dive into Neutralization Reactions
Mixing an acid and a base is a fundamental chemical process known as neutralization. Understanding this reaction is crucial in various fields, from everyday life to advanced chemical engineering. This comprehensive guide explores the intricacies of acid-base reactions, covering their mechanisms, applications, and the importance of safety precautions.
What are Acids and Bases?
Before diving into the mixing process, let's establish a clear understanding of acids and bases themselves. Several definitions exist, but the most common are the Arrhenius, Brønsted-Lowry, and Lewis definitions.
Arrhenius Definition
The Arrhenius definition, one of the earliest, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻). This definition is simple but limited in its scope. For example, it doesn't explain the basic properties of ammonia (NH₃), which doesn't contain hydroxide ions.
Brønsted-Lowry Definition
The Brønsted-Lowry definition offers a broader perspective. It defines acids as proton donors and bases as proton acceptors. This definition encompasses a wider range of substances, including ammonia, which can accept a proton. The key here is the transfer of a proton (H⁺).
Lewis Definition
The Lewis definition provides the most comprehensive description of acids and bases. It defines acids as electron pair acceptors and bases as electron pair donors. This definition includes reactions that don't involve protons, significantly expanding the scope of acid-base chemistry.
The Neutralization Reaction
When an acid and a base are mixed, they react to form salt and water. This is the essence of a neutralization reaction. The general equation is:
Acid + Base → Salt + Water
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) – common table salt – and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The reaction is essentially a combination of the H⁺ ion from the acid and the OH⁻ ion from the base to form water. The remaining ions then form the salt.
Types of Neutralization Reactions
Neutralization reactions aren't all the same. The specific products and the nature of the reaction depend on the strength of the acid and base involved.
Strong Acid-Strong Base Reactions
These reactions proceed essentially to completion, meaning nearly all the acid and base react to form salt and water. The resulting solution is generally neutral (pH 7), although slight deviations can occur depending on the concentrations and the nature of the salt formed. The reaction is highly exothermic, meaning it releases a significant amount of heat.
Weak Acid-Strong Base Reactions
These reactions don't go to completion. The equilibrium lies to the right, favoring the formation of salt and water, but a significant amount of the weak acid may remain unreacted. The resulting solution will be slightly basic (pH > 7) due to the presence of the conjugate base of the weak acid.
Strong Acid-Weak Base Reactions
Similar to weak acid-strong base reactions, these also don't proceed to completion. The equilibrium favors the formation of salt and water, but some of the weak base will remain unreacted. The resulting solution will be slightly acidic (pH < 7) because of the presence of the conjugate acid of the weak base.
Weak Acid-Weak Base Reactions
These reactions are the most complex. The extent of the reaction depends on the relative strengths of the weak acid and weak base. Predicting the pH of the resulting solution requires considering the equilibrium constants of both the acid and the base. The solution may be acidic, basic, or even neutral depending on the specific reactants.
Titration: Quantifying Neutralization
Titration is a crucial laboratory technique used to determine the concentration of an unknown acid or base solution. This process involves carefully adding a solution of known concentration (the titrant) to a solution of unknown concentration until the neutralization point is reached. This point, often indicated by a color change using an indicator, signifies that the moles of acid and base are stoichiometrically equivalent.
The data obtained from titration allows for the calculation of the unknown concentration using the equation:
M₁V₁ = M₂V₂
where:
- M₁ = molarity of the titrant
- V₁ = volume of the titrant
- M₂ = molarity of the unknown solution
- V₂ = volume of the unknown solution
Applications of Neutralization Reactions
Neutralization reactions are essential in numerous applications:
Industrial Processes
- Wastewater Treatment: Neutralization is crucial for treating industrial wastewater that contains acidic or basic pollutants. Adding a base to acidic wastewater or an acid to basic wastewater neutralizes the pH to environmentally safe levels.
- Chemical Synthesis: Many chemical syntheses involve neutralization reactions to control the pH and create desired products.
- Food and Beverage Industry: Neutralization is used to adjust the pH of various food and beverage products to improve their taste, texture, and stability.
Everyday Life
- Antacid Medications: Antacids contain bases that neutralize excess stomach acid, relieving heartburn and indigestion.
- Soap Making: Soap making involves the saponification process, where fats and oils react with a strong base (like lye) to produce soap and glycerol. This is essentially a neutralization reaction.
- Agriculture: Soil pH is crucial for plant growth. Farmers often use neutralization reactions to adjust the soil pH to optimal levels.
Safety Precautions
Working with acids and bases requires careful attention to safety:
- Protective Equipment: Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat.
- Dilution: Always add acid to water, never water to acid. This prevents dangerous splashing and heat generation.
- Ventilation: Ensure adequate ventilation to prevent exposure to harmful fumes.
- Disposal: Dispose of acid and base waste according to appropriate safety guidelines.
Conclusion
Mixing an acid and a base is a fundamental chemical reaction with far-reaching implications. Understanding the types of neutralization reactions, their applications, and the necessary safety precautions is crucial for anyone working with chemicals. From everyday uses like antacids to large-scale industrial processes like wastewater treatment, neutralization plays a significant role in our world. This detailed exploration hopefully illuminates the complexities and significance of this essential chemical process. Remember to always prioritize safety when handling acids and bases. Further research into specific acid-base reactions and their unique properties will enhance your understanding of this fascinating area of chemistry.
Latest Posts
Latest Posts
-
What Does A Positive Delta H Mean
Mar 31, 2025
-
What Holds An Ionic Bond Together
Mar 31, 2025
-
Genotype And Phenotype Punnett Square Examples
Mar 31, 2025
-
Equation Relating Electric Field And Voltage
Mar 31, 2025
-
Does A Plant Cell Have Dna
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Mixing An Acid And A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
