Mole Ratios For A Reaction Are Obtained From The
Muz Play
Mar 20, 2025 · 6 min read
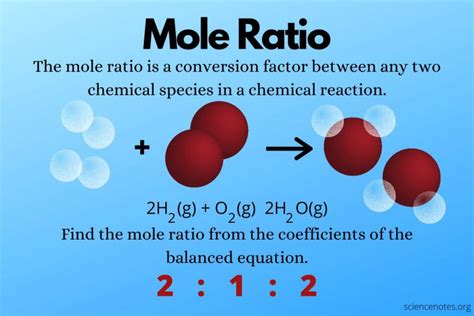
Table of Contents
Mole Ratios for a Reaction: Understanding the Coefficients in Balanced Chemical Equations
Mole ratios are fundamental to stoichiometry, the section of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. Understanding mole ratios is crucial for predicting the amount of product formed from a given amount of reactant or determining the amount of reactant needed to produce a desired amount of product. These ratios are directly derived from the coefficients in a balanced chemical equation. This article will delve deep into the concept of mole ratios, explaining their origin, application, and importance in various chemical calculations.
The Foundation: Balanced Chemical Equations
Before diving into mole ratios, it's essential to understand the significance of a balanced chemical equation. A balanced equation represents a chemical reaction where the number of atoms of each element is the same on both the reactant and product sides. This adherence to the law of conservation of mass is paramount. For example:
2H₂ + O₂ → 2H₂O
This balanced equation shows the reaction between hydrogen (H₂) and oxygen (O₂) to produce water (H₂O). The coefficients (the numbers in front of the chemical formulas) indicate the relative number of moles of each substance involved in the reaction. In this case:
- 2 moles of hydrogen gas react with
- 1 mole of oxygen gas to produce
- 2 moles of water
These coefficients are the key to determining mole ratios.
Extracting Mole Ratios from Balanced Equations
Mole ratios are simply the ratios of the coefficients in a balanced chemical equation. They represent the relative number of moles of reactants consumed and products produced. From the above example:
- Mole ratio of H₂ to O₂: 2:1 (Two moles of H₂ react with one mole of O₂)
- Mole ratio of H₂ to H₂O: 2:2 or 1:1 (Two moles of H₂ produce two moles of H₂O; this simplifies to a 1:1 ratio)
- Mole ratio of O₂ to H₂O: 1:2 (One mole of O₂ produces two moles of H₂O)
These ratios are crucial for performing stoichiometric calculations. They allow us to relate the amount of one substance in a reaction to the amount of another.
Applying Mole Ratios in Stoichiometric Calculations
Mole ratios form the backbone of all stoichiometric calculations. Let's illustrate their application with a few examples:
Example 1: Calculating the amount of product formed
Let's say we have 4 moles of hydrogen gas reacting with excess oxygen. How many moles of water will be produced?
Using the balanced equation (2H₂ + O₂ → 2H₂O) and the mole ratio of H₂ to H₂O (1:1), we can set up a proportion:
1 mole H₂ / 1 mole H₂O = 4 moles H₂ / x moles H₂O
Solving for x, we find that 4 moles of H₂ will produce 4 moles of H₂O.
Example 2: Determining the limiting reactant
Consider a reaction where 3 moles of hydrogen gas react with 2 moles of oxygen gas. Which reactant is the limiting reactant (the reactant that gets completely consumed, thus limiting the amount of product formed)?
First, we need to determine the mole ratio of H₂ to O₂ from the balanced equation (2H₂ + O₂ → 2H₂O): 2:1
Now, let's compare the mole ratio to the given moles of reactants:
- Hydrogen: 3 moles H₂ / 2 moles O₂ = 1.5
- Balanced equation ratio: 2 moles H₂ / 1 mole O₂ = 2
Since 1.5 < 2, there is less hydrogen relative to the balanced equation ratio. Therefore, hydrogen is the limiting reactant.
Example 3: Calculating the amount of reactant needed
Suppose we need to produce 6 moles of water. How many moles of oxygen gas are required?
Using the balanced equation (2H₂ + O₂ → 2H₂O) and the mole ratio of O₂ to H₂O (1:2), we set up a proportion:
1 mole O₂ / 2 moles H₂O = x moles O₂ / 6 moles H₂O
Solving for x, we find that 3 moles of oxygen gas are needed.
Beyond Simple Mole Ratios: More Complex Reactions
The principles discussed above apply equally well to more complex reactions involving multiple reactants and products. For instance, consider the combustion of propane:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Here, we can derive numerous mole ratios:
- C₃H₈ : O₂ = 1 : 5
- C₃H₈ : CO₂ = 1 : 3
- C₃H₈ : H₂O = 1 : 4
- O₂ : CO₂ = 5 : 3
- O₂ : H₂O = 5 : 4
- CO₂ : H₂O = 3 : 4
Each of these ratios can be used to perform stoichiometric calculations similar to the simpler examples shown earlier.
Incorporating Molar Mass for Mass-to-Mass Calculations
While mole ratios relate moles of reactants and products, often we work with masses. To bridge this gap, we use molar mass. Molar mass is the mass of one mole of a substance (grams/mole).
Let's revisit Example 1 but now with masses instead of moles:
Assume we have 72 grams of hydrogen gas (H₂ molar mass: 2 g/mol). How many grams of water will be produced?
-
Moles of H₂: 72 g H₂ / (2 g/mol) = 36 moles H₂
-
Moles of H₂O: Using the mole ratio (1:1), 36 moles H₂ will produce 36 moles H₂O.
-
Grams of H₂O: (36 moles H₂O) * (18 g/mol H₂O) = 648 g H₂O
Therefore, 72 grams of hydrogen gas will produce 648 grams of water.
Dealing with Percent Yield and Limiting Reactants
Real-world chemical reactions rarely achieve 100% yield due to various factors like incomplete reactions or side reactions. Percent yield accounts for this discrepancy:
Percent Yield = (Actual Yield / Theoretical Yield) * 100%
The theoretical yield is the calculated amount of product based on stoichiometry, while the actual yield is the experimentally obtained amount.
Limiting reactants, as previously discussed, influence the actual yield. The amount of product formed is determined by the limiting reactant, regardless of the amount of excess reactants present.
Advanced Applications of Mole Ratios
Mole ratios are not confined to simple chemical reactions. They extend their usefulness to various fields:
- Industrial Chemistry: Optimizing reaction conditions and predicting product yields in large-scale chemical processes.
- Environmental Science: Analyzing pollutant concentrations and assessing the environmental impact of chemical reactions.
- Biochemistry: Understanding metabolic pathways and enzyme kinetics, where mole ratios play a crucial role in determining reaction rates and product formation.
- Analytical Chemistry: Quantitative analysis using titration and other techniques rely heavily on mole ratios for accurate calculations.
Conclusion
Mole ratios, derived from the coefficients in balanced chemical equations, are the cornerstone of stoichiometry. Mastering the concept of mole ratios is essential for anyone studying or working in chemistry-related fields. Through understanding and applying these ratios, one can accurately predict the amount of reactants needed and products formed in a chemical reaction, optimizing processes and improving experimental design. From simple calculations to complex chemical analyses, the importance of mole ratios in chemical calculations cannot be overstated. The principles laid out here provide a strong foundation for tackling various stoichiometric problems and furthering your understanding of chemical reactions.
Latest Posts
Latest Posts
-
Used To Transfer Small Amounts Of Liquid
Mar 20, 2025
-
Addition And Subtraction Of Rational Expressions With Unlike Denominators
Mar 20, 2025
-
What Is Free Energy In Biology
Mar 20, 2025
-
Efield Due To A Point Charge
Mar 20, 2025
-
The Longitudinal Growth Of Long Bones Ceases When
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Mole Ratios For A Reaction Are Obtained From The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
