What Is Free Energy In Biology
Muz Play
Mar 20, 2025 · 7 min read
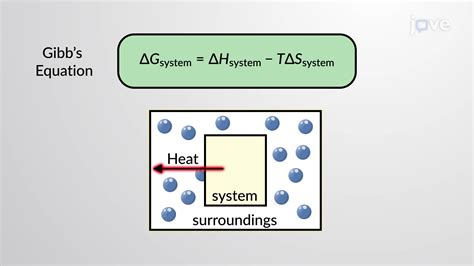
Table of Contents
What is Free Energy in Biology? A Deep Dive into Gibbs Free Energy and Biological Systems
Understanding free energy is crucial for grasping the fundamental principles governing biological processes. Life, at its core, is a complex interplay of energy transformations. From the synthesis of biomolecules to the intricate workings of cellular machinery, free energy dictates the spontaneity and directionality of these vital reactions. This article will delve into the concept of free energy in biology, focusing on Gibbs free energy and its applications in various biological systems.
Understanding Gibbs Free Energy: The Cornerstone of Biological Thermodynamics
In the realm of biological thermodynamics, Gibbs free energy (G) reigns supreme. It represents the amount of energy available in a system to perform useful work at a constant temperature and pressure. This is especially relevant to biological systems operating under relatively constant environmental conditions. The change in Gibbs free energy (ΔG) during a reaction dictates its spontaneity:
- ΔG < 0 (negative): The reaction is exergonic, meaning it releases free energy and proceeds spontaneously. This means the products have lower free energy than the reactants.
- ΔG > 0 (positive): The reaction is endergonic, requiring an input of free energy to proceed. The products have higher free energy than the reactants.
- ΔG = 0 (zero): The reaction is at equilibrium, with no net change in free energy. The rates of the forward and reverse reactions are equal.
The change in Gibbs free energy is determined by two primary factors:
- Enthalpy (H): Represents the total heat content of a system. Exothermic reactions (releasing heat, ΔH < 0) generally favor spontaneity.
- Entropy (S): Represents the disorder or randomness of a system. An increase in entropy (ΔS > 0) also favors spontaneity.
The relationship between these factors is elegantly expressed by the equation:
ΔG = ΔH - TΔS
where T is the absolute temperature in Kelvin. This equation highlights the interplay between enthalpy and entropy in determining the spontaneity of a reaction. A negative ΔG indicates a favorable reaction, regardless of whether it's driven primarily by a decrease in enthalpy or an increase in entropy.
Free Energy and Biological Reactions: A Closer Look
Biological reactions are rarely isolated events. Instead, they are often coupled, with an exergonic reaction driving an endergonic one. This coupling is essential for maintaining the complex and highly organized state of living organisms. A prime example is the coupling of ATP hydrolysis (an exergonic reaction) to drive endergonic reactions like protein synthesis or muscle contraction.
ATP (adenosine triphosphate): The "energy currency" of the cell, ATP hydrolysis releases a significant amount of free energy, making it an ideal energy source to power various cellular processes. The high-energy phosphate bonds in ATP are easily broken, releasing energy that can be harnessed for other reactions.
Free Energy and Metabolic Pathways
Metabolic pathways, the intricate networks of enzyme-catalyzed reactions within cells, are meticulously orchestrated to manage free energy effectively. These pathways are categorized into:
-
Catabolic pathways: These pathways break down complex molecules into simpler ones, releasing energy. These are generally exergonic processes, such as cellular respiration, where glucose is broken down to release energy stored as ATP. This energy release fuels numerous cellular activities.
-
Anabolic pathways: These pathways synthesize complex molecules from simpler ones, requiring energy input. These are endergonic processes, like protein synthesis, DNA replication, and the building of cellular structures. They rely on the energy released from catabolic pathways.
The regulation of metabolic pathways is crucial for maintaining cellular homeostasis and responding to environmental changes. Enzymes play a critical role in regulating the rate of reactions and thus controlling the flow of free energy through these pathways. Allosteric regulation, feedback inhibition, and other regulatory mechanisms fine-tune metabolic pathways to ensure efficient energy utilization and prevent wasteful energy expenditure.
Free Energy and Enzyme Catalysis
Enzymes are biological catalysts that accelerate the rate of biochemical reactions without being consumed themselves. They do this by lowering the activation energy, the energy barrier that must be overcome for a reaction to proceed. While enzymes do not alter the overall ΔG of a reaction, they dramatically speed up the reaction's rate, enabling biological processes to occur at the necessary pace for life. The efficiency of enzymes in harnessing and channeling free energy is vital for the smooth operation of metabolic pathways.
Free Energy in Specific Biological Processes: Examples
The concept of free energy is pervasive throughout biology, governing a wide range of processes. Let's explore a few examples:
1. Cellular Respiration: Harvesting Energy from Glucose
Cellular respiration is a prime example of an exergonic process. The stepwise breakdown of glucose through glycolysis, the citric acid cycle, and oxidative phosphorylation releases a substantial amount of free energy, a significant portion of which is captured in the form of ATP. The negative ΔG of each step ensures the overall spontaneity of the process. This energy is then used to power various cellular functions.
2. Photosynthesis: Capturing Solar Energy
Photosynthesis is an endergonic process that converts light energy into chemical energy in the form of glucose. Plants and other photosynthetic organisms utilize light energy to drive the synthesis of glucose from carbon dioxide and water. This process requires a considerable input of free energy, which is ultimately derived from sunlight. The careful orchestration of light-dependent and light-independent reactions ensures the efficient capture and utilization of solar energy.
3. Protein Synthesis: Building the Workhorses of the Cell
Protein synthesis is another example of an endergonic process. The assembly of amino acids into polypeptide chains requires a significant input of free energy, primarily driven by ATP hydrolysis. The accuracy and efficiency of this process are vital for the synthesis of functional proteins, which are essential for all aspects of cellular function. Errors in protein synthesis can have severe consequences.
4. Active Transport: Moving Molecules Against Their Concentration Gradients
Active transport mechanisms move molecules across cell membranes against their concentration gradients. This process requires energy input, as it increases the order (decreases entropy) of the system. ATP hydrolysis is a common energy source for active transport, driving the movement of ions or molecules against their concentration gradients. This process is crucial for maintaining cellular homeostasis and transporting essential molecules across membranes.
5. Muscle Contraction: Converting Chemical Energy into Mechanical Work
Muscle contraction is a complex process that involves the interaction of actin and myosin filaments. The sliding filament model explains how ATP hydrolysis provides the energy for the conformational changes in myosin that drive muscle contraction. The energy released from ATP hydrolysis is used to power the cyclical interactions between actin and myosin, leading to muscle shortening and generation of force.
Free Energy and the Second Law of Thermodynamics
The concept of free energy is intrinsically linked to the second law of thermodynamics, which states that the total entropy of an isolated system can only increase over time. Living organisms, however, appear to defy this law by maintaining a high degree of order and organization. This apparent contradiction is resolved by recognizing that living systems are not isolated; they constantly exchange energy and matter with their surroundings.
While living organisms maintain a low internal entropy, they increase the entropy of their surroundings through the release of heat and other waste products. The overall change in entropy (system + surroundings) remains positive, upholding the second law of thermodynamics. The ability to harness and utilize free energy allows living organisms to maintain a low internal entropy, creating and sustaining the complex structures and processes that define life.
Conclusion: The Importance of Free Energy in Biology
Free energy, particularly Gibbs free energy, is a cornerstone of biological thermodynamics, providing a framework for understanding the spontaneity and directionality of biological processes. From metabolic pathways to individual enzymatic reactions, free energy dictates the flow of energy within living systems. The ability to harness and utilize free energy is fundamental to the existence and maintenance of life, shaping the intricate network of biochemical reactions that sustain all living organisms. A comprehensive understanding of free energy is crucial for appreciating the complexity and elegance of biological systems. Further research into the dynamics of free energy within biological systems continues to unravel the mysteries of life itself.
Latest Posts
Latest Posts
-
Is Water Boiling A Physical Change
Mar 21, 2025
-
What Is A Point Charge In Physics
Mar 21, 2025
-
Una Maestra A Los Alumnos
Mar 21, 2025
-
In What Chapter Does Mary Wollstonecraft Talk About Female Labor
Mar 21, 2025
-
Is Melting Point Extensive Or Intensive
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is Free Energy In Biology . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
