Is Water Boiling A Physical Change
Muz Play
Mar 21, 2025 · 5 min read
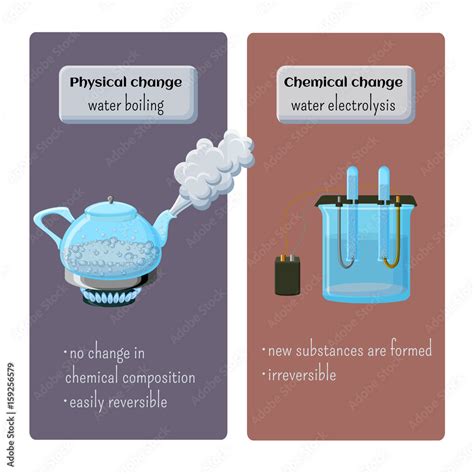
Table of Contents
Is Boiling Water a Physical Change? A Deep Dive into the Science
The question, "Is boiling water a physical change?" seems simple enough. But a truly comprehensive answer delves into the fundamental nature of matter, the differences between physical and chemical changes, and the specific properties of water. Let's explore this seemingly straightforward topic in detail.
Understanding Physical and Chemical Changes
Before we dissect the boiling of water, it's crucial to establish a clear understanding of the difference between physical and chemical changes. This distinction forms the bedrock of our investigation.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think about it this way: the fundamental building blocks of the substance remain the same; only their arrangement or state changes. Examples include:
- Melting ice: Ice (solid water) melts into liquid water. The water molecules are still H₂O; they've just transitioned from a rigid structure to a more mobile one.
- Crushing a can: The can's shape changes, but the aluminum remains aluminum.
- Dissolving sugar in water: The sugar disappears into the water, but its chemical structure remains intact. It can be recovered through evaporation.
Chemical Changes: Breaking and Forming Bonds
A chemical change, also known as a chemical reaction, involves a fundamental alteration in the chemical composition of a substance. New substances with different properties are formed, often accompanied by observable changes like heat release or gas production. Examples include:
- Burning wood: Wood reacts with oxygen, producing ash, smoke (various gases), and heat. The original wood is gone, transformed into entirely new substances.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a different compound with different properties.
- Baking a cake: The ingredients undergo chemical reactions, forming new compounds that give the cake its texture and flavor. The raw ingredients are fundamentally transformed.
Boiling Water: A Detailed Analysis
Now, let's focus on the act of boiling water. When you heat water, its temperature increases until it reaches its boiling point (100°C or 212°F at standard atmospheric pressure). At this point, a significant transformation occurs: the liquid water turns into water vapor (steam).
The key question is: does this transformation alter the chemical composition of the water? The answer is no.
The water molecules (H₂O) remain intact throughout the boiling process. No new chemical bonds are formed, and no existing bonds are broken. The change is purely a transition of state – from liquid to gas. The energy added through heating simply increases the kinetic energy of the water molecules, allowing them to overcome the intermolecular forces holding them together in the liquid phase. They become sufficiently energetic to escape into the gaseous phase as steam.
Evidence Supporting Physical Change:
- Reversible Process: Condensation, the reverse process of boiling, readily converts steam back into liquid water. This reversibility strongly suggests a physical change, as the original substance is recovered.
- Chemical Composition Remains Unchanged: The chemical formula of water remains H₂O before, during, and after boiling. No new substances are created. This is verifiable through various chemical tests.
- No New Substances Formed: No new chemical species appear. This contrasts sharply with chemical reactions where new compounds emerge.
- Observable Changes are Physical: The changes we observe – the bubbling, the formation of steam – are manifestations of the change in physical state, not a change in chemical identity.
Addressing Potential Counterarguments
While the evidence overwhelmingly supports boiling water as a physical change, some arguments might appear to challenge this conclusion. Let's address these:
The Decomposition of Water at Extremely High Temperatures
At extremely high temperatures (significantly beyond the typical boiling point), water molecules can decompose into their constituent elements: hydrogen (H₂) and oxygen (O₂). This is a chemical change, involving the breaking of the covalent bonds within the water molecule. However, this decomposition doesn't occur during normal boiling. The temperatures required are far beyond those encountered in everyday boiling processes.
Dissolved Impurities
Water often contains dissolved impurities. Boiling can concentrate these impurities as the water evaporates. While the concentration of impurities changes, this doesn't represent a chemical change in the water itself. The impurities remain chemically unchanged; their relative abundance merely shifts.
Changes in Isotopic Ratios
Water contains different isotopes of hydrogen and oxygen (e.g., deuterium instead of regular hydrogen). Boiling can subtly alter the ratios of these isotopes in the remaining water and the steam. However, this isotopic fractionation is a physical separation process, not a chemical transformation. The molecules remain water molecules; only their isotopic makeup changes slightly.
Beyond Boiling: Other Physical Changes in Water
Water demonstrates various physical changes besides boiling:
- Freezing: Liquid water transforms into solid ice.
- Melting: Solid ice turns into liquid water.
- Sublimation: Solid ice directly transforms into water vapor (occurs at low pressures).
- Deposition: Water vapor directly transforms into solid ice (occurs at low pressures).
- Vaporization (Evaporation): Liquid water slowly transforms into water vapor below the boiling point.
Conclusion: Boiling Water is a Physical Change
In conclusion, boiling water is unequivocally a physical change. The process solely involves a change of state from liquid to gas without any alteration in the chemical composition of the water itself. The water molecules retain their identity throughout the process. While extreme conditions can lead to chemical decomposition, this is far removed from the everyday experience of boiling water. Understanding this distinction is fundamental to comprehending the basic principles of chemistry and the behavior of matter. The seemingly simple act of boiling water offers a compelling illustration of the fascinating world of physical changes.
Latest Posts
Latest Posts
-
In General Pathogens Grow Very Slowly
Mar 28, 2025
-
Mixing An Acid And A Base
Mar 28, 2025
-
Difference Between Meiosis 1 And Meiosis 2
Mar 28, 2025
-
How To Find The Hydronium Ion Concentration
Mar 28, 2025
-
What Is The Mass Of A Proton In Amu
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Water Boiling A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
