What Is The Mass Of A Proton In Amu
Muz Play
Mar 28, 2025 · 5 min read
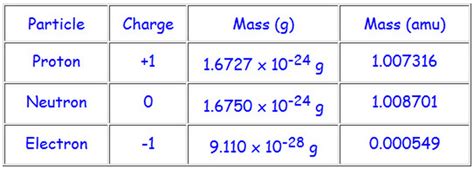
Table of Contents
What is the Mass of a Proton in amu? A Deep Dive into Atomic Mass Units and Proton Properties
The seemingly simple question, "What is the mass of a proton in amu?" opens a door to a fascinating exploration of atomic structure, measurement units, and the fundamental building blocks of matter. While the answer itself is straightforward, understanding its implications requires delving into the intricacies of atomic mass units (amu) and the properties of protons.
Understanding Atomic Mass Units (amu)
Before we address the proton's mass, let's clarify the unit of measurement: the atomic mass unit (amu), also known as the dalton (Da). This unit is crucial in understanding the mass of atoms and subatomic particles. It's defined as one twelfth (1/12) of the mass of a single unbound neutral atom of carbon-12. This seemingly arbitrary choice provides a convenient and consistent standard for comparing the masses of various atoms and subatomic particles.
The key here is the relative nature of amu. We're not measuring the mass in kilograms or grams, but rather comparing the mass of a particle to the mass of a well-defined standard—carbon-12. This allows us to express the masses of protons, neutrons, and other subatomic particles in a way that's easy to understand and compare. Since the mass of a single proton is relatively small compared to macroscopic units, amu provides a much more practical scale.
The Mass of a Proton in amu: The Answer and its Nuances
The mass of a proton is approximately 1.007276 amu. However, it's important to note that this is an average value. The actual mass of a proton can vary slightly depending on factors such as its environment and energy state. These variations are incredibly small, but they exist and are significant in high-precision measurements.
Furthermore, the value is an experimental determination. Scientists have employed various sophisticated techniques, including mass spectrometry, to precisely measure the proton's mass. These measurements continually refine our understanding, leading to slight adjustments in the accepted value over time. The number 1.007276 amu represents the currently accepted consensus, but future research may yield even more precise figures.
Why isn't the proton's mass exactly 1 amu?
The fact that a proton's mass is not exactly 1 amu reflects the complexities of nuclear physics. The mass of a proton isn't simply the sum of its constituent quarks. According to the Standard Model of particle physics, protons are composed of three quarks: two up quarks and one down quark. However, the combined mass of these quarks accounts for only a small fraction of the proton's total mass. The majority of the proton's mass arises from the strong nuclear force, which binds the quarks together. This force generates a substantial amount of energy, which, according to Einstein's famous equation, E=mc², contributes significantly to the proton's mass. This energy contribution is often referred to as binding energy.
The concept of mass-energy equivalence is crucial here. The strong force's energy isn't just some abstract concept; it directly contributes to the proton's observable mass. This is a fundamental principle of modern physics, highlighting the interconnectedness of mass and energy.
Beyond the Simple Answer: Exploring Related Concepts
Understanding the proton's mass in amu opens the door to a broader understanding of related atomic and nuclear concepts. Let's explore some of these:
1. Neutron Mass: The mass of a neutron is also expressed in amu and is very close to that of a proton. Its mass is approximately 1.008665 amu. The slight difference between the masses of protons and neutrons contributes to the stability of various atomic nuclei.
2. Isotopes and Atomic Mass: The concept of isotopes is directly related to the mass of protons and neutrons. Isotopes of an element have the same number of protons (defining the element) but differ in the number of neutrons. This variation in neutron number leads to different atomic masses for the isotopes of a single element. The atomic mass reported on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element.
3. Mass Spectrometry: Mass spectrometry is a powerful analytical technique that precisely measures the mass-to-charge ratio of ions. It's extensively used in various scientific fields, including chemistry, biology, and materials science, to identify and quantify different molecules and isotopes. The development and refinement of mass spectrometry have been instrumental in accurately determining the mass of protons and other subatomic particles.
4. Nuclear Binding Energy: As mentioned earlier, a significant portion of a proton's mass comes from the binding energy of the strong nuclear force. This binding energy is related to the stability of the nucleus. Nuclei with higher binding energies per nucleon (proton or neutron) are more stable. This concept is central to understanding nuclear reactions, such as nuclear fusion and fission.
5. Applications in various fields: The precise mass of a proton has crucial implications in various scientific and technological domains. This knowledge is fundamental to:
* **Nuclear Physics:** Understanding nuclear reactions and the stability of atomic nuclei.
* **Particle Physics:** Studying the fundamental constituents of matter and their interactions.
* **Cosmology:** Modeling the formation and evolution of the universe.
* **Chemistry:** Determining the composition and properties of molecules.
* **Medical Imaging:** Techniques like PET (Positron Emission Tomography) rely on understanding the behavior of subatomic particles.
Conclusion: A Journey into the Atomic World
The mass of a proton in amu, while seemingly a simple number (approximately 1.007276 amu), represents a profound achievement in scientific understanding. It's not merely a numerical value but a cornerstone upon which many important concepts in physics and chemistry are built. Understanding the nuances of atomic mass units, the contribution of binding energy, and the relationship between mass and energy provides a deeper appreciation for the complexities and elegance of the atomic world. Furthermore, continuous research and advancements in measurement techniques constantly refine our understanding, providing increasingly precise values and insights into the fundamental building blocks of matter.
Latest Posts
Latest Posts
-
Who Created The Law Of Conservation Of Mass
Mar 31, 2025
-
What Does A Positive Delta H Mean
Mar 31, 2025
-
What Holds An Ionic Bond Together
Mar 31, 2025
-
Genotype And Phenotype Punnett Square Examples
Mar 31, 2025
-
Equation Relating Electric Field And Voltage
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Of A Proton In Amu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
