Who Created The Law Of Conservation Of Mass
Muz Play
Mar 31, 2025 · 5 min read
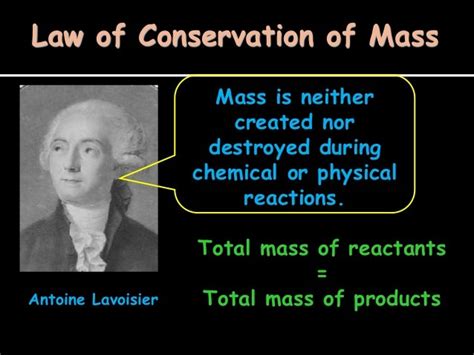
Table of Contents
Who Created the Law of Conservation of Mass? A Deep Dive into its History and Development
The Law of Conservation of Mass, a cornerstone of chemistry and physics, states that mass is neither created nor destroyed in a chemical reaction. While seemingly simple, the understanding and articulation of this fundamental principle involved the contributions of numerous scientists over centuries. Pinpointing a single "creator" is therefore inaccurate; instead, we should explore the evolution of this concept, highlighting the key players who shaped our understanding.
Early Glimpses and Alchemical Influences
The concept of conservation, albeit vaguely, can be traced back to ancient civilizations. Alchemists, though often operating with mystical interpretations, implicitly recognized the persistence of matter during transformations. Their experiments, while lacking the rigor of modern science, involved manipulating substances and observing changes. Although they didn't articulate it formally, the consistent weights of materials before and after their procedures, even if interpreted through a different lens, hinted at the concept of mass conservation. However, the lack of precise measurement tools and a systematic approach prevented them from formulating a clear scientific law.
The Dawn of Quantitative Chemistry: Lavoisier's Crucial Role
While alchemists provided foundational observations, it was Antoine-Laurent Lavoisier, a French chemist considered the "father of modern chemistry," who played a pivotal role in establishing the Law of Conservation of Mass. His meticulous experimental work and emphasis on quantitative measurements were revolutionary.
Lavoisier's Experimental Approach: A Paradigm Shift
Unlike his predecessors, Lavoisier conducted experiments with unprecedented precision. He used a carefully sealed vessel to track the mass of reactants and products in chemical reactions. This groundbreaking approach allowed him to directly observe that the total mass remained constant throughout the process. His experiments on combustion, notably the burning of phosphorus and sulfur in air, demonstrated that the increase in mass of the product was due to the combination of the substance with a component of the air, which he later identified as oxygen.
The Birth of a Law: Publication and Recognition
Lavoisier’s meticulous experiments and rigorous data analysis provided strong empirical evidence for the conservation of mass. He clearly articulated this principle in his seminal work, Traité Élémentaire de Chimie (Elementary Treatise on Chemistry), published in 1789. This publication not only presented the Law of Conservation of Mass but also established a systematic nomenclature for chemical substances, laying the foundation for modern chemical terminology. Lavoisier's work was instrumental in shifting chemistry from a qualitative to a quantitative science. While he didn't discover the law itself, his crucial contribution was formalizing it, providing compelling experimental evidence, and establishing it as a fundamental principle of chemistry.
Before Lavoisier: Precursors and Influences
It's important to acknowledge that Lavoisier didn't operate in a vacuum. Several scientists before him contributed to the development of the concept, although their work was often less precise and lacked the same level of systematic investigation.
Mikhail Lomonosov: A Russian Pioneer
Mikhail Lomonosov, a renowned Russian scientist, polymath, and poet, conducted experiments in the mid-18th century that suggested the conservation of mass. His work, which involved meticulously weighing materials before and after heating in sealed vessels, revealed that the total mass remained constant. Although his findings were not as widely disseminated as Lavoisier's, they anticipated the later formalization of the law. The language barrier and the political climate of the time might have limited the recognition of his important contributions.
Other Early Contributors
Various other scientists throughout the 17th and 18th centuries made incremental contributions to the understanding of mass conservation. These contributions, though often isolated or less systematic, collectively paved the way for Lavoisier's breakthrough. Their work highlights the collaborative and incremental nature of scientific discovery.
Refining the Law: Addressing Limitations and Exceptions
The Law of Conservation of Mass, as formulated by Lavoisier, held true for most ordinary chemical reactions. However, the discovery of radioactivity and nuclear reactions in the late 19th and early 20th centuries presented a significant challenge. In nuclear reactions, a small amount of mass is converted into energy according to Einstein's famous equation, E=mc². This seemingly contradicts the law of conservation of mass, as mass isn't strictly conserved.
The Birth of the Law of Conservation of Mass-Energy
Einstein's theory of special relativity showed that mass and energy are interchangeable. This led to the formulation of the Law of Conservation of Mass-Energy, which states that the total amount of mass and energy in a closed system remains constant. This broader law incorporates the exceptions to the original Law of Conservation of Mass, making it a more comprehensive and accurate principle.
Beyond the Laboratory: The Law's Impact
The Law of Conservation of Mass, despite its limitations in the realm of nuclear physics, remains a fundamental principle in chemistry. It serves as a powerful tool in:
- Stoichiometry: Calculating the amounts of reactants and products in chemical reactions.
- Chemical Equation Balancing: Ensuring that the number of atoms of each element is equal on both sides of a chemical equation.
- Understanding Chemical Processes: Providing a framework for understanding the transformations of matter during chemical reactions.
The law's significance extends beyond its practical applications. It represents a paradigm shift in scientific thinking, demonstrating the power of meticulous observation and quantitative analysis in understanding natural phenomena.
Conclusion: A Collective Effort
In conclusion, attributing the creation of the Law of Conservation of Mass to a single individual is an oversimplification. While Antoine-Laurent Lavoisier is rightfully recognized for his crucial role in formalizing the law through meticulous experimentation and clear articulation, his work built upon the earlier insights of alchemists and scientists like Mikhail Lomonosov. The law's evolution highlights the collaborative and incremental nature of scientific progress, where many individuals contribute to the development of a significant scientific principle. The later refinements, incorporating Einstein's theory of relativity, show that scientific laws are not immutable truths but rather models that are constantly refined and expanded as our understanding of the universe deepens. The law continues to be a cornerstone of chemistry and physics, reminding us of the enduring principles governing the transformations of matter in our world.
Latest Posts
Latest Posts
-
Examples Of Instantaneous Rate Of Change
Apr 01, 2025
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
-
What Does A Negative Reduction Potential Mean
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Who Created The Law Of Conservation Of Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
