How To Find The Hydronium Ion Concentration
Muz Play
Mar 28, 2025 · 6 min read
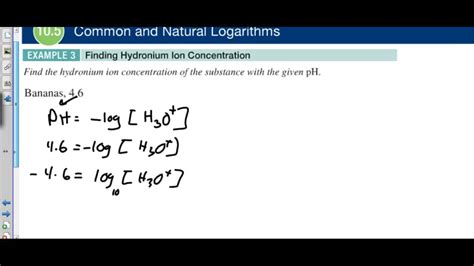
Table of Contents
How to Find the Hydronium Ion Concentration: A Comprehensive Guide
Determining the hydronium ion concentration, [H₃O⁺], is a fundamental concept in chemistry, crucial for understanding acidity, basicity, and numerous chemical reactions. This concentration dictates the pH of a solution, a measure of its acidity or alkalinity. This comprehensive guide will explore various methods for calculating [H₃O⁺], from simple strong acid solutions to more complex scenarios involving weak acids, weak bases, and buffer solutions. We'll also delve into the relationship between [H₃O⁺], pH, and pOH, equipping you with the knowledge to tackle a wide range of problems.
Understanding Hydronium Ions and pH
Before diving into the calculations, let's solidify our understanding of the basics. Hydronium ions (H₃O⁺) are formed when a proton (H⁺), a hydrogen ion, is transferred to a water molecule (H₂O). While we often represent acid dissociation as releasing H⁺, it's more accurate to depict it as forming H₃O⁺. The concentration of these hydronium ions directly relates to the acidity of a solution.
The pH of a solution is defined as the negative logarithm (base 10) of the hydronium ion concentration:
pH = -log₁₀[H₃O⁺]
A lower pH indicates a higher [H₃O⁺] and thus a more acidic solution. Conversely, a higher pH signifies a lower [H₃O⁺] and a more alkaline (basic) solution. A pH of 7 indicates neutrality at 25°C.
Calculating [H₃O⁺] for Strong Acids
Strong acids completely dissociate in water, meaning every molecule of the acid donates a proton to form a hydronium ion. This simplifies the calculation of [H₃O⁺].
Example: Calculate the [H₃O⁺] of a 0.1 M solution of hydrochloric acid (HCl).
HCl is a strong acid, and its dissociation is represented as:
HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)
Since HCl completely dissociates, the concentration of H₃O⁺ is equal to the initial concentration of HCl.
Therefore, [H₃O⁺] = 0.1 M
This simplicity extends to other strong monoprotic acids like nitric acid (HNO₃) and perchloric acid (HClO₄). For strong diprotic acids like sulfuric acid (H₂SO₄), the calculation is slightly more involved, as the first dissociation is complete, but the second is not. However, for many practical purposes, the [H₃O⁺] is approximated as twice the initial concentration of the acid.
Calculating [H₃O⁺] for Weak Acids
Weak acids only partially dissociate in water, meaning the equilibrium between the undissociated acid and its ions must be considered. This requires the use of the acid dissociation constant, Ka.
The general dissociation of a weak acid, HA, is:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
The acid dissociation constant, Ka, is expressed as:
Ka = [H₃O⁺][A⁻] / [HA]
To calculate [H₃O⁺], we often use an ICE (Initial, Change, Equilibrium) table.
Example: Calculate the [H₃O⁺] of a 0.1 M solution of acetic acid (CH₃COOH), given Ka = 1.8 x 10⁻⁵.
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| CH₃COOH | 0.1 | -x | 0.1 - x |
| H₃O⁺ | 0 | +x | x |
| CH₃COO⁻ | 0 | +x | x |
Substituting these equilibrium concentrations into the Ka expression:
1.8 x 10⁻⁵ = (x)(x) / (0.1 - x)
Since Ka is small, we can often approximate (0.1 - x) ≈ 0.1, simplifying the equation:
1.8 x 10⁻⁵ = x² / 0.1
Solving for x:
x = √(1.8 x 10⁻⁶) ≈ 1.34 x 10⁻³ M
Therefore, [H₃O⁺] ≈ 1.34 x 10⁻³ M
This approximation is valid when Ka is significantly smaller than the initial concentration of the weak acid. For cases where this approximation isn't valid, the quadratic formula must be used to solve for x.
Calculating [H₃O⁺] for Weak Bases
Weak bases react with water to produce hydroxide ions (OH⁻), which then react with water to form hydronium ions. The calculation involves the base dissociation constant, Kb, and the relationship between [H₃O⁺], [OH⁻], and Kw (the ion product constant of water).
Kw = [H₃O⁺][OH⁻] = 1.0 x 10⁻¹⁴ at 25°C
Example: Calculate the [H₃O⁺] of a 0.05 M solution of ammonia (NH₃), given Kb = 1.8 x 10⁻⁵.
First, find the [OH⁻] using an ICE table similar to the weak acid example, then use Kw to find [H₃O⁺].
Calculating [H₃O⁺] in Buffer Solutions
Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. They typically consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. The Henderson-Hasselbalch equation is useful for calculating [H₃O⁺] in buffer solutions:
pH = pKa + log₁₀([A⁻] / [HA])
where pKa = -log₁₀Ka.
Once you calculate the pH using the Henderson-Hasselbalch equation, you can then calculate [H₃O⁺] using the inverse logarithm:
[H₃O⁺] = 10⁻pH
The Importance of Considering Temperature
The Kw value is temperature-dependent. At temperatures other than 25°C, the Kw value will change, affecting the calculations of [H₃O⁺] and pH. For precise calculations at different temperatures, you'll need to use the appropriate Kw value for that temperature.
Advanced Scenarios and Complications
The methods described above cover the most common scenarios. However, more complex situations may arise, such as:
- Polyprotic acids: These acids can donate more than one proton. The calculation becomes more involved, requiring consideration of each dissociation step and its corresponding Ka value.
- Salt hydrolysis: Salts of weak acids or weak bases can affect the pH of a solution, requiring additional calculations to account for hydrolysis reactions.
- Ionic strength effects: In solutions with high ionic strength, the activity of ions deviates from their concentration, requiring activity coefficients to be considered for accurate calculations.
These more advanced scenarios often require iterative methods or specialized software for accurate calculations.
Conclusion: Mastering Hydronium Ion Concentration Calculations
Calculating the hydronium ion concentration is a cornerstone of acid-base chemistry. Understanding the different approaches—from the straightforward calculations for strong acids to the equilibrium considerations for weak acids and bases—is essential for anyone working in this field. Remember to consider the context of the problem, including the nature of the acid or base, the presence of a buffer, and the temperature, to select the appropriate method and ensure accurate results. By mastering these techniques, you'll gain a deeper understanding of solution chemistry and its implications across various scientific disciplines. Remember to practice regularly with diverse problems to solidify your understanding and develop your problem-solving skills. The more you practice, the more confident you'll become in navigating the complexities of hydronium ion concentration calculations.
Latest Posts
Latest Posts
-
What Are The Energy Yielding Nutrients
Mar 31, 2025
-
Angle For Bonds Ammonia And Water
Mar 31, 2025
-
Who Created The Law Of Conservation Of Mass
Mar 31, 2025
-
What Does A Positive Delta H Mean
Mar 31, 2025
-
What Holds An Ionic Bond Together
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Hydronium Ion Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
