Angle For Bonds Ammonia And Water
Muz Play
Mar 31, 2025 · 5 min read
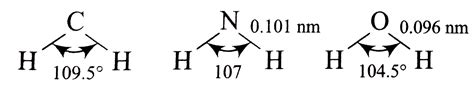
Table of Contents
Understanding Bond Angles: Ammonia (NH₃) and Water (H₂O)
Bond angles play a crucial role in determining the shapes of molecules and, consequently, their physical and chemical properties. Understanding these angles requires a grasp of valence shell electron pair repulsion (VSEPR) theory. This article delves deep into the bond angles of ammonia (NH₃) and water (H₂O), explaining the underlying principles and the factors influencing these angles. We'll explore the differences, similarities, and the implications of these variations.
The VSEPR Theory: A Foundation for Understanding Bond Angles
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone of molecular geometry prediction. It posits that electron pairs – both bonding and lone pairs – in the valence shell of a central atom repel each other. This repulsion leads to an arrangement that maximizes the distance between these electron pairs, thus defining the molecular geometry and, importantly, the bond angles. The ideal angles are achieved when electron pairs are positioned as far apart as possible to minimize repulsion. However, the presence of lone pairs introduces complexities.
Lone Pair-Lone Pair Repulsion
Lone pairs of electrons occupy more space than bonding pairs. This is because lone pairs are attracted only to one nucleus (the central atom), while bonding pairs are attracted to two nuclei (the central atom and the bonded atom). Consequently, lone pair-lone pair repulsion is stronger than lone pair-bonding pair repulsion, which in turn is stronger than bonding pair-bonding pair repulsion. This hierarchy of repulsions significantly influences the actual bond angles observed in molecules.
Ammonia (NH₃): A Tetrahedral Electron Geometry, Trigonal Pyramidal Molecular Geometry
Ammonia (NH₃) possesses a central nitrogen atom bonded to three hydrogen atoms. Nitrogen has five valence electrons: three are involved in bonding with hydrogen atoms, and two remain as a lone pair. According to VSEPR theory, this arrangement leads to a tetrahedral electron geometry. This means that the four electron pairs (three bonding pairs and one lone pair) are positioned as far apart as possible, ideally at 109.5°.
However, the presence of a lone pair alters the molecular geometry. The molecular geometry, which describes the arrangement of only the atoms, is trigonal pyramidal. The lone pair exerts a stronger repulsive force than the bonding pairs, pushing the hydrogen atoms slightly closer together.
The Bond Angle in Ammonia
The actual bond angle in ammonia is approximately 107°. This deviation from the ideal tetrahedral angle of 109.5° is directly attributable to the lone pair's greater spatial requirements and increased repulsive effect. The stronger repulsion from the lone pair compresses the H-N-H bond angle, making it smaller than the ideal tetrahedral angle.
Water (H₂O): Bent Molecular Geometry
Water (H₂O) also features a central atom (oxygen) surrounded by electron pairs. Oxygen has six valence electrons; two form single bonds with two hydrogen atoms, leaving two lone pairs. Similar to ammonia, the electron geometry is tetrahedral, based on the arrangement of four electron pairs around the central oxygen atom.
But again, the presence of lone pairs significantly influences the molecular geometry. In water, the molecular geometry is described as bent or V-shaped. The two lone pairs occupy more space than the bonding pairs, resulting in a smaller bond angle than the ideal tetrahedral angle.
The Bond Angle in Water
The actual bond angle in water is approximately 104.5°. This value is smaller than the 109.5° tetrahedral angle due to the increased repulsion exerted by the two lone pairs. The stronger repulsion from the two lone pairs pushes the hydrogen atoms even closer together than in ammonia, resulting in a smaller bond angle.
Comparing Bond Angles in Ammonia and Water
Both ammonia and water exhibit bond angles less than the ideal tetrahedral angle of 109.5°. This is a direct consequence of the presence of lone pairs. However, the magnitude of the deviation differs.
-
Water (104.5°) has a smaller bond angle than ammonia (107°). This difference arises from the presence of two lone pairs in water versus one in ammonia. The two lone pairs in water exert a stronger overall repulsive force on the bonding pairs, leading to a greater compression of the bond angle.
-
The greater influence of lone pairs in water explains its higher polarity compared to ammonia. This enhanced polarity affects water's physical properties such as its high boiling point, surface tension, and solvent abilities.
Factors Influencing Bond Angles: Beyond VSEPR
While VSEPR theory provides a good first approximation, other factors can subtly influence bond angles. These include:
-
Hybridization: The concept of orbital hybridization (sp³, sp², sp) further refines our understanding of bond angles. In both ammonia and water, the central atoms undergo sp³ hybridization, contributing to the tetrahedral electron geometry. However, the presence of lone pairs modifies the ideal angles dictated by pure hybridization.
-
Electronegativity: The electronegativity differences between the central atom and the bonded atoms can have a small effect on bond angles. A higher electronegativity difference might slightly alter the electron distribution, influencing the repulsions and thus the angle. However, this effect is usually less significant than the effects of lone pairs.
-
Steric Effects: In larger molecules with bulky substituents, steric hindrance (repulsion between atoms or groups that are not directly bonded) can play a role in determining bond angles.
Conclusion: Bond Angles and Molecular Properties
Understanding the bond angles in ammonia and water is crucial for comprehending their diverse physical and chemical properties. VSEPR theory provides an excellent framework for predicting these angles, while recognizing the influence of lone pairs, hybridization, and other subtle factors. The smaller bond angles in these molecules compared to the ideal tetrahedral angle result directly from the stronger repulsions exerted by lone pairs of electrons. This understanding extends beyond simple molecular geometry; it forms the foundation for explaining the unique behaviors of these crucial molecules in chemical reactions and biological systems. The difference in bond angles between ammonia and water highlights the significant role of the number of lone pairs and their impact on molecular shape and properties. This knowledge is fundamental in chemistry, impacting fields like materials science, biochemistry, and environmental science.
Latest Posts
Latest Posts
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
-
What Does A Negative Reduction Potential Mean
Apr 01, 2025
-
How To Calculate Standard Free Energy Change
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Angle For Bonds Ammonia And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
