Molecules Are Related To Matter Because
Muz Play
Mar 17, 2025 · 6 min read
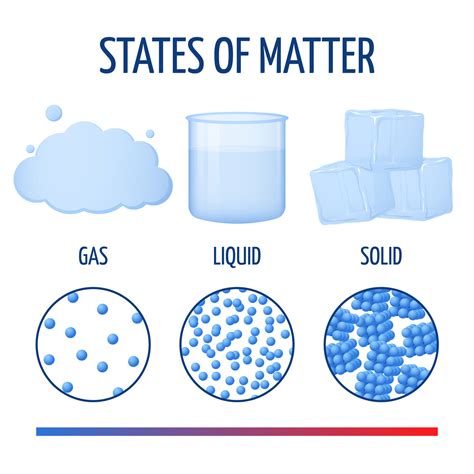
Table of Contents
Molecules Are Related to Matter Because… They Are Matter!
The relationship between molecules and matter isn't simply a connection; it's a fundamental identity. Matter, anything that occupies space and has mass, is fundamentally composed of molecules (and, more fundamentally, atoms). Understanding this relationship unlocks a deeper understanding of the physical world around us, from the smallest particles to the largest structures. This article delves into the intricate connection between molecules and matter, exploring their various forms, properties, and interactions.
Defining Matter and Molecules
Before exploring their relationship, let's define our key terms.
Matter: The Stuff of the Universe
Matter encompasses everything that possesses both mass and volume. This includes solids, liquids, gases, and plasma – the four fundamental states of matter. From the air we breathe to the ground we walk on, and everything in between, all are composed of matter. The mass of matter represents its resistance to acceleration, while its volume indicates the space it occupies.
Molecules: The Building Blocks of Matter
Molecules are groups of two or more atoms held together by chemical bonds. These atoms can be of the same element (like in oxygen gas, O₂) or different elements (like in water, H₂O). Molecules are the fundamental units of many chemical compounds and play a crucial role in determining the properties of matter. The arrangement, type, and number of atoms within a molecule profoundly influence its behavior and interactions.
The crucial link: Molecules are the building blocks of matter. In other words, matter is composed of molecules. Different arrangements and types of molecules create diverse forms of matter, leading to the vast array of materials we observe in the universe.
The Diverse Forms of Matter and Their Molecular Structure
The relationship between molecules and matter is further illuminated by exploring the various states of matter and how molecular structure influences them.
Solids: Strong Molecular Bonds and Ordered Structures
In solids, molecules are tightly packed together, often in a highly ordered crystalline structure. The strong intermolecular forces restrict molecular movement, resulting in a rigid and definite shape. The properties of a solid, like hardness, melting point, and conductivity, are directly related to the type of molecules involved and the strength of the bonds between them. For example, diamonds, composed of carbon atoms in a strong, tightly bonded lattice structure, are incredibly hard, while ice, with its less densely packed water molecules, is relatively soft.
Liquids: Moderate Molecular Bonds and Fluid Movement
Liquids exhibit a less ordered structure than solids. Molecules in liquids are still close together, but they possess more freedom of movement, allowing the liquid to flow and take the shape of its container. The intermolecular forces in liquids are weaker than in solids, accounting for their fluidity. The viscosity (thickness) of a liquid is influenced by the shape and size of its molecules and the strength of their interactions.
Gases: Weak Molecular Bonds and Random Movement
In gases, molecules are widely dispersed and move randomly at high speeds. The intermolecular forces are weak, allowing gas molecules to occupy a large volume and be easily compressed. The behavior of gases is described by the gas laws, which relate pressure, volume, temperature, and the number of gas molecules. The lightness or heaviness of a gas is directly tied to the mass of its constituent molecules.
Plasma: Ionized Gases and Unique Properties
Plasma, often considered the fourth state of matter, consists of highly ionized gas where electrons are stripped from atoms, forming a mixture of ions and free electrons. This results in unique electrical conductivity and responsiveness to magnetic fields. Plasmas are found in stars, lightning bolts, and neon lights, demonstrating a highly dynamic state of matter deeply linked to its underlying molecular (and atomic) composition.
Molecular Properties and Matter's Characteristics
The properties of matter are directly determined by the properties of the molecules that compose it. Let's explore some key examples:
Color: Molecular Absorption and Reflection of Light
The color of matter is determined by how its molecules interact with light. Molecules absorb certain wavelengths of light and reflect others. The reflected wavelengths determine the color we perceive. For instance, chlorophyll molecules in plants absorb most wavelengths except green, resulting in the green color we associate with plants. Similarly, the vibrant colors of gemstones arise from the way their constituent molecules interact with light.
Odor: Volatile Molecules and Olfactory Receptors
The odor or smell of a substance is related to the volatility of its molecules. Volatile molecules readily evaporate and travel through the air to reach our olfactory receptors in the nose. The specific shape and chemical composition of these molecules influence how they interact with our olfactory receptors, resulting in the perception of different smells. Fragrances are meticulously designed based on our understanding of how molecular structure relates to scent perception.
Taste: Molecular Interactions with Taste Buds
Taste, similar to smell, depends on the interaction of molecules with our taste buds. Different molecules interact with specific receptors on our tongue, leading to the perception of sweet, sour, salty, bitter, and umami tastes. The shape and charge of molecules are key factors in determining how they bind to taste receptors and, thus, the taste experienced.
Reactivity: Chemical Bonds and Molecular Transformations
The reactivity of matter refers to its tendency to undergo chemical changes. This reactivity is a direct consequence of the molecular structure and the types of chemical bonds present. Molecules with weak bonds are more likely to react than those with strong bonds. Chemical reactions involve the breaking and formation of chemical bonds, leading to the transformation of molecules and the formation of new substances. This explains why some materials are highly reactive (like sodium metal) while others are inert (like noble gases).
Advanced Concepts: Macromolecules and Matter's Complexity
Moving beyond simple molecules, we encounter macromolecules, which are incredibly large molecules composed of thousands or even millions of atoms. These play a crucial role in the complexity of living matter.
Polymers: Long Chains of Repeating Units
Polymers, such as DNA, proteins, and plastics, are formed by the repeated linking of smaller molecules called monomers. The properties of polymers are strongly influenced by the type of monomers and how they are arranged. The immense variety of polymers accounts for the diversity of materials found in both living organisms and synthetic materials.
Proteins: Complex Structures with Diverse Functions
Proteins, essential components of living organisms, are macromolecules with complex three-dimensional structures. Their functions are highly dependent on their precise molecular structure, which determines how they interact with other molecules. Enzymes, antibodies, and structural proteins all exemplify the intricate relationship between a molecule's structure and its function within the context of living matter.
Nucleic Acids: Information Carriers in Living Systems
Nucleic acids, such as DNA and RNA, are macromolecules that carry genetic information. The sequence of nucleotides in DNA determines the genetic code, which dictates the synthesis of proteins and other macromolecules. The precise arrangement of molecules within these nucleic acids is directly responsible for heredity and the functioning of life itself.
Conclusion: An Inseparable Relationship
The relationship between molecules and matter is not merely associative; it's foundational. Matter, in all its diverse forms, is inherently composed of molecules, whose properties dictate the characteristics of that matter. From the simplest gases to the most complex living organisms, molecules are the building blocks, the architects, and the functional units that shape the physical world as we know it. Understanding this fundamental relationship is critical for advancing our knowledge in fields ranging from materials science and chemistry to biology and medicine. The study of molecules continues to reveal the incredible complexity and beauty inherent in the structure and behavior of matter.
Latest Posts
Latest Posts
-
The Gram Stain Acid Fast Stain And Endospore Stain
Mar 17, 2025
-
How To Determine Melting Point Of Compounds
Mar 17, 2025
-
What Is The Relationship Between Force And Acceleration
Mar 17, 2025
-
Minerals Are Formed By The Process Of
Mar 17, 2025
-
M7 9 3 Perimeters And Areas Of Comp Fig
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Molecules Are Related To Matter Because . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
