Movement Of Energy During Phase Transitions
Muz Play
Mar 19, 2025 · 6 min read
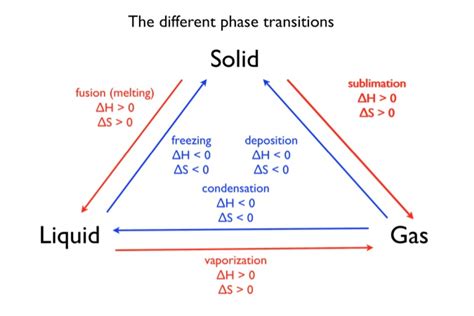
Table of Contents
Movement of Energy During Phase Transitions
Phase transitions are fundamental processes in nature, involving a change in the physical state of a substance, such as melting ice (solid to liquid), boiling water (liquid to gas), or depositing vapor (gas to solid). These transitions are accompanied by significant energy changes, and understanding the movement of energy during these processes is crucial in various fields, from materials science and engineering to meteorology and climate modeling. This article delves deep into the mechanics of energy transfer involved in phase transitions, exploring the concepts of latent heat, enthalpy, entropy, and their interrelationships.
Understanding Phase Transitions: A Microscopic Perspective
Before diving into the energetics, let's briefly review the microscopic picture. A substance exists in a specific phase due to the strength of intermolecular forces between its constituent particles (atoms or molecules).
-
Solid Phase: In solids, these forces are strong, holding particles in a rigid, ordered structure. Particles vibrate around fixed positions, but their movement is limited.
-
Liquid Phase: In liquids, intermolecular forces are weaker than in solids, allowing particles to move more freely, resulting in a less ordered structure with a definite volume but no fixed shape.
-
Gas Phase: In gases, intermolecular forces are significantly weak, allowing particles to move independently with high kinetic energy, resulting in neither a fixed shape nor a fixed volume.
A phase transition involves a shift in the balance of these intermolecular forces and the kinetic energy of the particles. Adding or removing energy influences this balance, driving the transition from one phase to another.
The Role of Latent Heat: The Hidden Energy
The most striking feature of phase transitions is the absorption or release of energy without a change in temperature. This energy is known as latent heat, a term highlighting its "hidden" nature. It represents the energy required to overcome the intermolecular forces holding the substance in its current phase.
Latent Heat of Fusion: Solid to Liquid
The latent heat of fusion (L<sub>f</sub>) is the energy needed to change one unit mass of a substance from solid to liquid at its melting point. This energy is used to break the strong bonds holding the particles in the solid's ordered structure. For instance, when ice melts, the energy absorbed overcomes the hydrogen bonds between water molecules, allowing them to transition to the more mobile liquid state.
Latent Heat of Vaporization: Liquid to Gas
The latent heat of vaporization (L<sub>v</sub>) is the energy needed to change one unit mass of a substance from liquid to gas at its boiling point. This energy is significantly higher than the latent heat of fusion because it requires overcoming even stronger intermolecular forces to completely separate the particles and transform them into the gaseous state. When water boils, energy input overcomes the hydrogen bonding and allows water molecules to escape the liquid surface, forming steam.
Latent Heat of Sublimation: Solid to Gas
Sublimation is the direct transition from a solid to a gas without passing through the liquid phase (e.g., dry ice). The latent heat of sublimation (L<sub>s</sub>) is the energy needed to directly change one unit mass of a substance from solid to gas. This energy accounts for both breaking the solid's structure and overcoming intermolecular forces to achieve gaseous dispersion.
Enthalpy: The Total Heat Content
While latent heat focuses on the energy required for phase changes, enthalpy (H) encompasses the total heat content of a system at constant pressure. It includes both the internal energy of the system (kinetic and potential energy of particles) and the energy related to the system's volume and pressure. The change in enthalpy (ΔH) during a phase transition is directly related to the latent heat:
-
ΔH<sub>fusion</sub> = m * L<sub>f</sub> (where m is the mass)
-
ΔH<sub>vaporization</sub> = m * L<sub>v</sub>
-
ΔH<sub>sublimation</sub> = m * L<sub>s</sub>
These equations highlight that the enthalpy change during a phase transition is directly proportional to the mass of the substance undergoing the transition and the respective latent heat.
Entropy: The Measure of Disorder
Entropy (S) is a thermodynamic property representing the degree of randomness or disorder within a system. Phase transitions invariably involve a change in entropy. When a substance transitions from a solid to a liquid or a liquid to a gas, its entropy increases, reflecting the increased disorder of the particles. This increase in entropy is driven by the energy input during the transition, which allows particles to overcome the constraints of their ordered structure.
The change in entropy (ΔS) during a phase transition is related to the heat absorbed (q) and the absolute temperature (T) by:
ΔS = q/T
This relationship highlights that the entropy change is directly proportional to the heat absorbed and inversely proportional to the absolute temperature. The higher the temperature, the less the entropy change for a given amount of heat.
Gibbs Free Energy: Predicting Spontaneity
Gibbs free energy (G) combines enthalpy and entropy to determine the spontaneity of a process. It is defined as:
G = H - TS
The change in Gibbs free energy (ΔG) during a phase transition indicates whether the transition will occur spontaneously at a given temperature and pressure.
-
ΔG < 0: The transition is spontaneous.
-
ΔG > 0: The transition is non-spontaneous; the reverse transition is favored.
-
ΔG = 0: The transition is at equilibrium; the rates of the forward and reverse transitions are equal.
At the phase transition temperature, ΔG = 0, indicating equilibrium between the two phases.
Applications and Real-World Examples
Understanding the movement of energy during phase transitions is crucial in numerous applications:
-
Climate modeling: Evaporation, condensation, melting, and freezing of water are key processes in weather patterns and climate change. The latent heat involved significantly influences atmospheric temperatures and energy transport.
-
Materials science: Phase transitions play a critical role in materials processing, influencing properties like strength, ductility, and electrical conductivity. Controlling the energy input during phase transitions is essential for creating materials with desired properties.
-
Refrigeration and air conditioning: These technologies rely on phase transitions of refrigerants, using the absorption or release of latent heat to achieve cooling.
-
Power generation: Steam turbines in power plants utilize the latent heat of vaporization of water to generate electricity.
-
Food preservation: Freezing food relies on the removal of latent heat to lower its temperature below that of microbial growth.
Conclusion: A Dynamic Interplay of Energy and Order
The movement of energy during phase transitions is a complex and fascinating phenomenon that governs many natural and technological processes. The concepts of latent heat, enthalpy, entropy, and Gibbs free energy provide a powerful framework for understanding the energy changes and spontaneity of these transitions. Further research continues to refine our understanding of these processes, leading to advancements in various fields. From predicting weather patterns to designing new materials, mastering the principles of energy transfer during phase transitions remains a cornerstone of scientific and technological progress. The interplay between energy input, molecular interactions, and the resulting changes in order and disorder define the fundamental nature of phase transitions and their wide-ranging impact.
Latest Posts
Latest Posts
-
Inverted Vs Everted Palindromic Dna Sequence Example
Mar 19, 2025
-
What Makes Up Most Of The Mass Of An Atom
Mar 19, 2025
-
Policy Implementation Refers To The Bureaucratic Function Of
Mar 19, 2025
-
Example Of A Formal Lab Report For Chemistry
Mar 19, 2025
-
The Fluid Filled Area Within The Chloroplast Is Called The
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Movement Of Energy During Phase Transitions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
