Neutralization Reaction Of Naoh And Hcl
Muz Play
Mar 26, 2025 · 5 min read
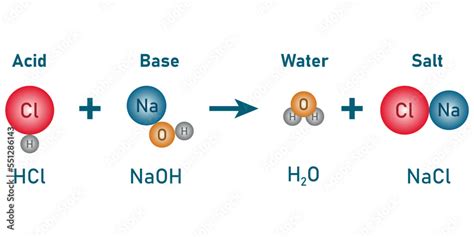
Table of Contents
Neutralization Reaction of NaOH and HCl: A Deep Dive
The reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction provides a strong foundation for grasping acid-base chemistry, stoichiometry, and titration techniques. This comprehensive guide delves into the specifics of this reaction, exploring its mechanism, applications, and implications.
Understanding Acids and Bases
Before diving into the specifics of the NaOH and HCl reaction, let's establish a clear understanding of acids and bases. Several definitions exist, but the most relevant for this discussion are the Arrhenius and Brønsted-Lowry definitions.
Arrhenius Definition
According to Arrhenius, an acid is a substance that produces hydrogen ions (H⁺) when dissolved in water, while a base produces hydroxide ions (OH⁻). HCl, a strong acid, readily dissociates in water to form H⁺ and Cl⁻ ions:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
NaOH, a strong base, similarly dissociates to yield Na⁺ and OH⁻ ions:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Brønsted-Lowry Definition
The Brønsted-Lowry definition broadens the scope. An acid is a proton (H⁺) donor, and a base is a proton acceptor. This definition encompasses more substances than the Arrhenius definition, particularly in non-aqueous solutions. In the reaction between HCl and NaOH, HCl acts as the acid (donating a proton), and NaOH acts as the base (accepting the proton).
The Neutralization Reaction: NaOH + HCl
The neutralization reaction between NaOH and HCl is a double displacement reaction where the hydrogen ions (H⁺) from the acid react with the hydroxide ions (OH⁻) from the base to form water (H₂O). The other product is a salt, sodium chloride (NaCl), which remains dissolved in the solution.
The balanced chemical equation is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This equation represents the complete ionic equation. To understand the net ionic equation, we need to break down the strong electrolytes into their constituent ions:
Na⁺(aq) + OH⁻(aq) + H⁺(aq) + Cl⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
The spectator ions, Na⁺ and Cl⁻, appear on both sides of the equation and do not participate directly in the reaction. Therefore, the net ionic equation simplifies to:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation highlights the essence of the neutralization reaction: the combination of H⁺ and OH⁻ ions to form water. This reaction is highly exothermic, meaning it releases heat.
Stoichiometry and Calculations
Stoichiometry allows us to quantify the reactants and products involved in chemical reactions. For the NaOH and HCl reaction, the balanced equation shows a 1:1 mole ratio between NaOH and HCl. This means that one mole of NaOH reacts completely with one mole of HCl.
For example, if we have 0.1 moles of NaOH, we would need 0.1 moles of HCl for complete neutralization. This principle is crucial in titrations, a technique used to determine the concentration of an unknown solution using a solution of known concentration.
Titration Calculations
Titration involves carefully adding a standard solution (a solution of known concentration) to a solution of unknown concentration until the reaction is complete. The point at which the reaction is complete is called the equivalence point. In the NaOH and HCl titration, the equivalence point is typically determined using an indicator that changes color at a specific pH.
To calculate the concentration of an unknown NaOH solution using a standardized HCl solution, we can use the following formula derived from the stoichiometry of the reaction:
M₁V₁ = M₂V₂
Where:
- M₁ = Molarity of the HCl solution (known)
- V₁ = Volume of the HCl solution used (measured)
- M₂ = Molarity of the NaOH solution (unknown)
- V₂ = Volume of the NaOH solution (measured)
By measuring the volume of HCl needed to neutralize a known volume of NaOH, we can calculate the concentration of the NaOH solution.
Applications of the Neutralization Reaction
The neutralization reaction between NaOH and HCl has numerous applications across various fields:
1. Acid-Base Titrations
As mentioned earlier, this reaction is fundamental to acid-base titrations, which are used extensively in analytical chemistry to determine the concentration of unknown acid or base solutions. This is vital in various industries, from pharmaceutical manufacturing to environmental monitoring.
2. pH Control
Neutralization reactions are crucial for controlling pH levels in various processes. For instance, in wastewater treatment, NaOH might be used to neutralize acidic wastewater before discharge. Similarly, HCl can be used to neutralize alkaline waste. Maintaining the correct pH is essential for environmental protection and preventing corrosion of pipes and equipment.
3. Chemical Synthesis
The reaction is used in many chemical syntheses where a specific pH needs to be maintained or a specific salt, like NaCl, is required as a byproduct. The careful control of the reaction conditions is crucial for yield and purity.
4. Digestion of Samples
In analytical chemistry, the neutralization reaction can aid in the digestion of samples. For instance, some organic matter or minerals might require treatment with acids or bases before analysis.
5. Food and Beverage Industry
NaOH and HCl find applications in food processing, though not necessarily in a direct neutralization reaction. NaOH is often used in the production of certain food items, while HCl is a component of some food preservatives and regulates pH in various food and beverage processes.
Safety Precautions
Working with strong acids and bases like NaOH and HCl requires careful attention to safety. Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. Handle the chemicals cautiously, avoiding direct contact with skin or eyes. Neutralization reactions can be exothermic, so ensure adequate ventilation to prevent the buildup of heat. Dispose of waste chemicals according to the proper guidelines. Proper handling and disposal are extremely important for environmental protection.
Conclusion
The neutralization reaction between NaOH and HCl is a cornerstone of acid-base chemistry. Its simplicity belies its profound importance in various scientific and industrial applications. Understanding the stoichiometry, the net ionic equation, and the safety precautions involved is vital for anyone working with these chemicals. From quantitative analysis in titrations to controlling pH in industrial processes, this reaction plays a crucial role in numerous contexts. Further exploration into related acid-base concepts, such as pH, buffers, and indicators, will enhance a deeper comprehension of chemical reactivity and its practical relevance. The study of this reaction provides a solid foundation for advancing knowledge in chemistry and related fields.
Latest Posts
Latest Posts
-
How To Determine If A Reaction Is Spontaneous
Mar 29, 2025
-
What Bonds Are The Most Polar
Mar 29, 2025
-
Systems Of Linear Equations And Inequalities
Mar 29, 2025
-
Cuanto Pesa Un Galon De Agua
Mar 29, 2025
-
Example Of The First Law Of Thermodynamics
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Neutralization Reaction Of Naoh And Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
